Patterning of the Drosophila retina by the morphogenetic furrow
- PMID: 37091979
- PMCID: PMC10117938
- DOI: 10.3389/fcell.2023.1151348
Patterning of the Drosophila retina by the morphogenetic furrow
Abstract
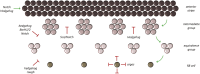
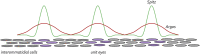
Pattern formation is the process by which cells within a homogeneous epithelial sheet acquire distinctive fates depending upon their relative spatial position to each other. Several proposals, starting with Alan Turing's diffusion-reaction model, have been put forth over the last 70 years to describe how periodic patterns like those of vertebrate somites and skin hairs, mammalian molars, fish scales, and avian feather buds emerge during development. One of the best experimental systems for testing said models and identifying the gene regulatory networks that control pattern formation is the compound eye of the fruit fly, Drosophila melanogaster. Its cellular morphogenesis has been extensively studied for more than a century and hundreds of mutants that affect its development have been isolated. In this review we will focus on the morphogenetic furrow, a wave of differentiation that takes an initially homogeneous sheet of cells and converts it into an ordered array of unit eyes or ommatidia. Since the discovery of the furrow in 1976, positive and negative acting morphogens have been thought to be solely responsible for propagating the movement of the furrow across a motionless field of cells. However, a recent study has challenged this model and instead proposed that mechanical driven cell flow also contributes to retinal pattern formation. We will discuss both models and their impact on patterning.
Keywords: Drosophila; cell flow; diffusion-reaction; eye; morphogen; morphogenetic furrow; pattern formation; positional information.
Copyright © 2023 Warren and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Extramacrochaetae imposes order on the Drosophila eye by refining the activity of the Hedgehog signaling gradient.Development. 2013 May;140(9):1994-2004. doi: 10.1242/dev.088963. Epub 2013 Mar 27. Development. 2013. PMID: 23536565 Free PMC article.
-
The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave.Development. 1991 Nov;113(3):841-50. doi: 10.1242/dev.113.3.841. Development. 1991. PMID: 1726564
-
Drosophila eye development: Notch and Delta amplify a neurogenic pattern conferred on the morphogenetic furrow by scabrous.Mech Dev. 1995 Feb;49(3):173-89. doi: 10.1016/0925-4773(94)00314-d. Mech Dev. 1995. PMID: 7734391
-
Genetics of epithelial polarity and pattern in the Drosophila retina.Bioessays. 1999 Apr;21(4):275-85. doi: 10.1002/(SICI)1521-1878(199904)21:4<275::AID-BIES3>3.0.CO;2-5. Bioessays. 1999. PMID: 10377890 Review.
-
My what big eyes you have: how the Drosophila retina grows.Dev Neurobiol. 2011 Dec;71(12):1133-52. doi: 10.1002/dneu.20921. Dev Neurobiol. 2011. PMID: 21604387 Free PMC article. Review.
Cited by
-
miR-137 targets Myc to regulate growth during eye development.Development. 2025 Jul 15;152(14):dev204373. doi: 10.1242/dev.204373. Epub 2025 Jul 16. Development. 2025. PMID: 40554764 Free PMC article.
-
Deep conservation complemented by novelty and innovation in the insect eye ground plan.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2416562122. doi: 10.1073/pnas.2416562122. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793041 Free PMC article.
-
EyeHex toolbox for complete segmentation of ommatidia in fruit fly eyes.Biol Open. 2025 Jun 15;14(6):bio061962. doi: 10.1242/bio.061962. Epub 2025 Jun 23. Biol Open. 2025. PMID: 40549456 Free PMC article.
References
-
- Abaturova M. P., Ginter E. K. (1968). The transplantation of the imaginal discs of the mutation ophtalmopedia in Drosophila melanogaster . Genetika 4, 58–64.
-
- Auerbach C. (1936). The development of the legs, wings, and halteres in wild type and some mutant strains of Drosophila melanogaster . Trans. R. Soc. Edin. LVIII. Part III, No. 27.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

