Granulosa cell-derived extracellular vesicles mitigate the detrimental impact of thermal stress on bovine oocytes and embryos
- PMID: 37091982
- PMCID: PMC10116072
- DOI: 10.3389/fcell.2023.1142629
Granulosa cell-derived extracellular vesicles mitigate the detrimental impact of thermal stress on bovine oocytes and embryos
Abstract
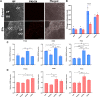
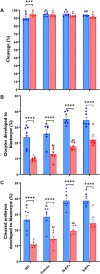
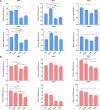
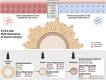
Climate change-induced global warming results in rises in body temperatures above normal physiological levels (hyperthermia) with negative impacts on reproductive function in dairy and beef animals. Extracellular vesicles (EVs), commonly described as nano-sized, lipid-enclosed complexes, harnessed with a plethora of bioactive cargoes (RNAs, proteins, and lipids), are crucial to regulating processes like folliculogenesis and the initiation of different signaling pathways. The beneficial role of follicular fluid-derived EVs in inducing thermotolerance to oocytes during in vitro maturation (IVM) has been evidenced. Here we aimed to determine the capacity of in vitro cultured granulosa cell-derived EVs (GC-EVs) to modulate bovine oocytes' thermotolerance to heat stress (HS) during IVM. Moreover, this study tested the hypothesis that EVs released from thermally stressed GCs (S-EVs) shuttle protective messages to provide protection against subsequent HS in bovine oocytes. For this, sub-populations of GC-EVs were generated from GCs subjected to 38.5°C (N-EVs) or 42°C (S-EVs) and supplemented to cumulus-oocyte complexes (COCs) matured in vitro at the normal physiological body temperature of the cow (38.5°C) or HS (41°C) conditions. Results indicate that S-EVs improve the survival of oocytes by reducing ROS accumulation, improving mitochondrial function, and suppressing the expression of stress-associated genes thereby reducing the severity of HS on oocytes. Moreover, our findings indicate a carryover impact from the addition of GC-EVs during oocyte maturation in the development to the blastocyst stage with enhanced viability.
Keywords: cumulus-oocyte complex; embryo development; extracellular vesicles; granulosa cells; heat stress; oocyte maturation.
Copyright © 2023 Menjivar, Gad, Gebremedhn, Ghosh and Tesfaye.
Conflict of interest statement
Author SaG was employed by Genus Plc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Extracellular vesicles shuttle protective messages against heat stress in bovine granulosa cells.Sci Rep. 2020 Sep 25;10(1):15824. doi: 10.1038/s41598-020-72706-z. Sci Rep. 2020. PMID: 32978452 Free PMC article.
-
Extracellular vesicles of follicular fluid from heat-stressed cows modify the gene expression of in vitro-matured oocytes.Anim Reprod Sci. 2019 Jun;205:94-104. doi: 10.1016/j.anireprosci.2019.04.008. Epub 2019 Apr 24. Anim Reprod Sci. 2019. PMID: 31060922
-
Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development.PLoS One. 2017 Jun 15;12(6):e0179451. doi: 10.1371/journal.pone.0179451. eCollection 2017. PLoS One. 2017. PMID: 28617821 Free PMC article.
-
Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation.Hum Reprod Update. 2016 Mar-Apr;22(2):182-93. doi: 10.1093/humupd/dmv055. Epub 2015 Dec 9. Hum Reprod Update. 2016. PMID: 26663221 Free PMC article. Review.
-
The effects and mechanisms of heat stress on mammalian oocyte and embryo development.J Therm Biol. 2024 Aug;124:103927. doi: 10.1016/j.jtherbio.2024.103927. Epub 2024 Aug 3. J Therm Biol. 2024. PMID: 39153259 Review.
Cited by
-
Mapping the follicle-specific regulation of extracellular vesicle-mediated microRNA transport in the southern white rhinoceros (Ceratotherium simum simum)†.Biol Reprod. 2024 Aug 15;111(2):376-390. doi: 10.1093/biolre/ioae081. Biol Reprod. 2024. PMID: 38775197 Free PMC article.
-
Rumen-mammary gland axis and bacterial extracellular vesicles: Exploring a new perspective on heat stress in dairy cows.Anim Nutr. 2024 Sep 16;19:70-75. doi: 10.1016/j.aninu.2024.08.003. eCollection 2024 Dec. Anim Nutr. 2024. PMID: 39628643 Free PMC article. Review.
-
Bovine oviductal organoids: a multi-omics approach to capture the cellular and extracellular molecular response of the oviduct to heat stress.BMC Genomics. 2023 Oct 27;24(1):646. doi: 10.1186/s12864-023-09746-y. BMC Genomics. 2023. PMID: 37891479 Free PMC article.
-
Advancements in Genetic Biomarkers and Exogenous Antioxidant Supplementation for Safeguarding Mammalian Cells against Heat-Induced Oxidative Stress and Apoptosis.Antioxidants (Basel). 2024 Feb 20;13(3):258. doi: 10.3390/antiox13030258. Antioxidants (Basel). 2024. PMID: 38539792 Free PMC article. Review.
-
Extracellular vesicles in dairy cattle: research progress and prospects for practical applications.J Anim Sci Biotechnol. 2025 Aug 4;16(1):110. doi: 10.1186/s40104-025-01242-5. J Anim Sci Biotechnol. 2025. PMID: 40754626 Free PMC article. Review.
References
-
- Abdelnour S. A., Yang C. Y., Swelum A. A., Abd El-Hack M. E., Khafaga A. F., Abdo M., et al. (2020). Molecular, functional, and cellular alterations of oocytes and cumulus cells induced by heat stress and shock in animals. Environ. Sci. Pollut. Res. Int. 27 (31), 38472–38490. 10.1007/s11356-020-10302-4 - DOI - PubMed
-
- Abumaghaid M. M., Abdelazim A. M., Belali T. M., Alhujaily M., Saadeldin I. M. (2022). Shuttle transfer of mRNA transcripts via extracellular vesicles from male reproductive tract cells to the cumulus-oocyte complex in rabbits (Oryctolagus cuniculus). Front. Veterinary Sci. 9, 816080. 10.3389/fvets.2022.816080 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

