Implications of Tau Dysregulation in Huntington's Disease and Potential for New Therapeutics
- PMID: 37092231
- PMCID: PMC10200226
- DOI: 10.3233/JHD-230569
Implications of Tau Dysregulation in Huntington's Disease and Potential for New Therapeutics
Abstract
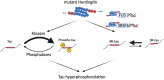
Huntington's disease (HD) is an autosomal dominant neurodegenerative disorder. The disease, characterized by motor, cognitive, and psychiatric impairments, is caused by the expansion of a CAG repeat in the huntingtin gene. Despite the discovery of the mutation in 1993, no disease-modifying treatments are yet available. Understanding the molecular and cellular mechanisms involved in HD is therefore crucial for the development of novel treatments. Emerging research has found that HD might be classified as a secondary tauopathy, with the presence of tau insoluble aggregates in late HD. Increased total tau protein levels have been observed in both HD patients and animal models of HD. Tau hyperphosphorylation, the main feature of tau pathology, has also been investigated and our own published results suggest that the protein phosphorylation machinery is dysregulated in the early stages of HD in R6/1 transgenic mice, primarily in the cortex and striatum. Protein phosphorylation, catalysed by kinases, regulates numerous cellular mechanisms and has been shown to be dysregulated in other neurodegenerative disorders, including Alzheimer's disease. While it is still unclear how the mutation in the huntingtin gene leads to tau dysregulation in HD, several hypotheses have been explored. Evidence suggests that the mutant huntingtin does not directly interact with tau, but instead interacts with tau kinases, phosphatases, and proteins involved in tau alternative splicing, which could result in tau dysregulation as observed in HD. Altogether, there is increasing evidence that tau is undergoing pathological changes in HD and may be a good therapeutic target.
Keywords: Huntington’s disease; aggregates; phosphorylation; tau; tauopathy.
Conflict of interest statement
The authors have no competing interests to disclose.
Figures

Similar articles
-
Tau hyperphosphorylation and deregulation of calcineurin in mouse models of Huntington's disease.Hum Mol Genet. 2015 Jan 1;24(1):86-99. doi: 10.1093/hmg/ddu456. Epub 2014 Sep 8. Hum Mol Genet. 2015. PMID: 25205109
-
Faulty splicing and cytoskeleton abnormalities in Huntington's disease.Brain Pathol. 2016 Nov;26(6):772-778. doi: 10.1111/bpa.12430. Brain Pathol. 2016. PMID: 27529534 Free PMC article. Review.
-
Downregulation of glial genes involved in synaptic function mitigates Huntington's disease pathogenesis.Elife. 2021 Apr 19;10:e64564. doi: 10.7554/eLife.64564. Elife. 2021. PMID: 33871358 Free PMC article.
-
Treatment with Tau fibrils impact Huntington's disease-related phenotypes in cell and mouse models.Neurobiol Dis. 2024 Nov;202:106696. doi: 10.1016/j.nbd.2024.106696. Epub 2024 Oct 9. Neurobiol Dis. 2024. PMID: 39389154
-
Role of Phosphorylated Tau and Glucose Synthase Kinase 3 Beta in Huntington's Disease Progression.J Alzheimers Dis. 2019;72(s1):S177-S191. doi: 10.3233/JAD-190851. J Alzheimers Dis. 2019. PMID: 31744007 Review.
Cited by
-
Protein modification in neurodegenerative diseases.MedComm (2020). 2024 Aug 4;5(8):e674. doi: 10.1002/mco2.674. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39105197 Free PMC article. Review.
-
Mild cognitive impairment in Huntington's disease: challenges and outlooks.J Neural Transm (Vienna). 2024 Apr;131(4):289-304. doi: 10.1007/s00702-024-02744-8. Epub 2024 Jan 24. J Neural Transm (Vienna). 2024. PMID: 38265518 Review.
-
The Search for a Universal Treatment for Defined and Mixed Pathology Neurodegenerative Diseases.Int J Mol Sci. 2024 Dec 14;25(24):13424. doi: 10.3390/ijms252413424. Int J Mol Sci. 2024. PMID: 39769187 Free PMC article. Review.
-
Mutant-Huntingtin Molecular Pathways Elucidate New Targets for Drug Repurposing.Int J Mol Sci. 2023 Nov 27;24(23):16798. doi: 10.3390/ijms242316798. Int J Mol Sci. 2023. PMID: 38069121 Free PMC article. Review.
-
Concomitant pathologies and their impact on Huntington's disease. A brief review of current evidence.J Neural Transm (Vienna). 2025 Jun 21. doi: 10.1007/s00702-025-02957-5. Online ahead of print. J Neural Transm (Vienna). 2025. PMID: 40542842 Review. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

