The aromatic Claisen rearrangement of a 1,2-azaborine
- PMID: 37092259
- PMCID: PMC10175209
- DOI: 10.1039/d2ob02186b
The aromatic Claisen rearrangement of a 1,2-azaborine
Abstract
The first aromatic Claisen rearrangement of a 1,2-azaborine is described along with a quantitative kinetic comparison of the reaction of the azaborine with its direct all-carbon analogue. The azaborine A rearranged in a clean, regioselective fashion and reacted faster than the all-carbon substrate B at all temperatures from 140-180 °C. Activation free energies were extracted from observed first-order rate constants (A: ΔG‡298K = 32.7 kcal mol-1; B: ΔG‡298K = 34.8 kcal mol-1) corresponding to a twenty fold faster rate for A at observed reaction temperatures. DFT calculations show that the rearrangement proceeds via a concerted six-membered transition state and that the electronic structure of the BN and CC rings is mostly responsible for the observed regioselectivity and relative reactivity.
Conflict of interest statement
Conflicts of interest
The authors declare no competing financial interest.
Figures

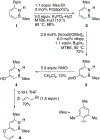
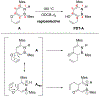
References
-
- (a) Bosdet MJ and Piers WE, Can. J. Chem, 2009, 87, 8–29;
- Liu Z and Marder T, Angew. Chem., Int. Ed, 2008, 47, 242–244; - PubMed
- Campbell PG, Marwitz AJV and Liu S-Y, Angew. Chem., Int. Ed, 2012, 51, 6074–6092; - PMC - PubMed
- Giustra ZX and Liu SY, J. Am. Chem. Soc, 2018, 140, 1184–1194. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

