Plant secondary metabolite citral interferes with Phytophthora capsici virulence by manipulating the expression of effector genes
- PMID: 37092279
- PMCID: PMC10346372
- DOI: 10.1111/mpp.13340
Plant secondary metabolite citral interferes with Phytophthora capsici virulence by manipulating the expression of effector genes
Abstract
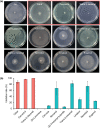
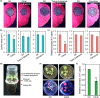
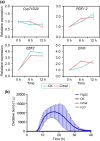
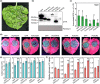
Phytophthora capsici is a notorious pathogen that infects various economically important plants and causes serious threats to agriculture worldwide. Plants deploy a variety of plant secondary metabolites to fend off pathogen attacks, but the molecular mechanisms are largely unknown. In this study, we screened 11 plant secondary metabolites to evaluate their biofumigation effects against P. capsici, and found that citral, carvacrol, and trans-2-decenal exhibited strong antimicrobial effects. Intriguingly, a low concentration of citral was effective in restricting P. capsici infection in Nicotiana benthamiana, but it was unable to inhibit the mycelial growth. A high concentration of citral affected the mycelial growth and morphology, zoospore germination, and cell membrane permeability of P. capsici. Further investigations showed that citral did not induce expression of tested plant immunity-related genes and reactive oxygen species (ROS) production, suggesting that a low concentration of citral could not trigger plant immunity. Moreover, RNA-Seq analysis showed that citral treatment regulated the expression of some P. capsici effector genes such as RxLR genes and P. cactorum-fragaria (PCF)/small cysteine-rich (SCR)74-like genes during the infection process, which was also verified by reverse transcription-quantitative PCR assay. Five candidate effector genes suppressed by citral significantly facilitated P. capsici infection in N. benthamiana or inhibited ROS triggered by flg22, suggesting that they were virulence factors of P. capsici. Together, our results revealed that plant-derived citral exhibited excellent inhibitory efficacy against P. capsici by suppressing vegetative growth and manipulating expression of effector genes, which provides a promising application of citral for controlling Phytophthora blight.
Keywords: Phytophthora blight; antimicrobial activity; mechanism; plant secondary metabolite; virulence gene.
© 2023 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Figures



Similar articles
-
Inhibitory activity and antioomycete mechanism of citral against Phytophthora capsici.Pestic Biochem Physiol. 2024 Sep;204:106067. doi: 10.1016/j.pestbp.2024.106067. Epub 2024 Aug 2. Pestic Biochem Physiol. 2024. PMID: 39277383
-
A Virulence Essential CRN Effector of Phytophthora capsici Suppresses Host Defense and Induces Cell Death in Plant Nucleus.PLoS One. 2015 May 26;10(5):e0127965. doi: 10.1371/journal.pone.0127965. eCollection 2015. PLoS One. 2015. PMID: 26011314 Free PMC article.
-
RNAi-Based Gene Silencing of RXLR Effectors Protects Plants Against the Oomycete Pathogen Phytophthora capsici.Mol Plant Microbe Interact. 2022 Jun;35(6):440-449. doi: 10.1094/MPMI-12-21-0295-R. Epub 2022 Apr 26. Mol Plant Microbe Interact. 2022. PMID: 35196108
-
The oomycete broad-host-range pathogen Phytophthora capsici.Mol Plant Pathol. 2012 May;13(4):329-37. doi: 10.1111/j.1364-3703.2011.00754.x. Epub 2011 Oct 20. Mol Plant Pathol. 2012. PMID: 22013895 Free PMC article. Review.
-
Effectors of Phytophthora pathogens are powerful weapons for manipulating host immunity.Planta. 2019 Aug;250(2):413-425. doi: 10.1007/s00425-019-03219-x. Epub 2019 Jun 26. Planta. 2019. PMID: 31243548 Review.
Cited by
-
Harnessing Plant's Arsenal: Essential Oils as Promising Tools for Sustainable Management of Potato Late Blight Disease Caused by Phytophthora infestans-A Comprehensive Review.Molecules. 2023 Oct 27;28(21):7302. doi: 10.3390/molecules28217302. Molecules. 2023. PMID: 37959721 Free PMC article. Review.
References
-
- Cadena, M.B. , Preston, G.M. , Van der Hoorn, R.A.L. , Townley, H.E. & Thompson, I.P. (2018) Species‐specific antimicrobial activity of essential oils and enhancement by encapsulation in mesoporous silica nanoparticles. Industrial Crops and Products, 122, 582–590.
-
- Dudai, N. , Weinstein, Y. , Krup, M. , Rabinski, T. & Ofir, R. (2005) Citral is a new inducer of caspase‐3 in tumor cell lines. Planta Medica, 71, 484–488. - PubMed
-
- Dudareva, N. , Negre, F. , Nagegowda, D.A. & Orlova, I. (2006) Plant volatiles: recent advances and future perspectives. Critical Reviews in Plant Sciences, 25, 417–440.
-
- Dunn, A.R. , Milgroom, M.G. , Meitz, J.C. , McLeod, A. , Fry, W.E. , McGrath, M.T. et al. (2010) Population structure and resistance to mefenoxam of Phytophthora capsici in New York state. Plant Disease, 94, 1461–1468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

