Research Progress on Biomimetic Nanomaterials for Electrochemical Glucose Sensors
- PMID: 37092419
- PMCID: PMC10123724
- DOI: 10.3390/biomimetics8020167
Research Progress on Biomimetic Nanomaterials for Electrochemical Glucose Sensors
Abstract
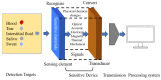
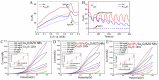
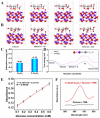
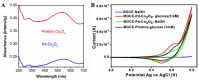
Diabetes has become a chronic disease that necessitates timely and accurate detection. Among various detection methods, electrochemical glucose sensors have attracted much attention because of low cost, real-time detection, and simple and easy operation. Nonenzymatic biomimetic nanomaterials are the vital part in electrochemical glucose sensors. This review article summarizes the methods to enhance the glucose sensing performance of noble metal, transition metal oxides, and carbon-based materials and introduces biomimetic nanomaterials used in noninvasive glucose detection in sweat, tear, urine, and saliva. Based on these, this review provides the foundation for noninvasive determination of trace glucose for diabetic patients in the future.
Keywords: biomimetic nanomaterials; electrochemical; glucose sensing; noninvasive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Electrochemical nonenzymatic sensing of glucose using advanced nanomaterials.Mikrochim Acta. 2017 Dec 13;185(1):49. doi: 10.1007/s00604-017-2609-1. Mikrochim Acta. 2017. PMID: 29594566 Review.
-
Recent progress on nanomaterial-based electrochemical sensors for glucose detection in human body fluids.Mikrochim Acta. 2025 Jan 29;192(2):110. doi: 10.1007/s00604-025-06972-x. Mikrochim Acta. 2025. PMID: 39878884 Review.
-
Nanomaterials-based electrochemical sensors for the detection of natural antioxidants in food and biological samples: research progress.Mikrochim Acta. 2022 Aug 5;189(9):318. doi: 10.1007/s00604-022-05403-5. Mikrochim Acta. 2022. PMID: 35931898 Review.
-
Current advancements and prospects of enzymatic and non-enzymatic electrochemical glucose sensors.Int J Biol Macromol. 2023 Dec 31;253(Pt 2):126680. doi: 10.1016/j.ijbiomac.2023.126680. Epub 2023 Sep 4. Int J Biol Macromol. 2023. PMID: 37673151 Review.
-
Nanostructured Transition Metal Sulfide-based Glucose and Lactic Acid Electrochemical Sensors for Clinical Applications.Curr Top Med Chem. 2023;23(4):284-294. doi: 10.2174/1568026623666221205093154. Curr Top Med Chem. 2023. PMID: 36475346 Review.
Cited by
-
Bio-Inspired Nanomaterials for Micro/Nanodevices: A New Era in Biomedical Applications.Micromachines (Basel). 2023 Sep 18;14(9):1786. doi: 10.3390/mi14091786. Micromachines (Basel). 2023. PMID: 37763949 Free PMC article. Review.
-
Two-Dimensional Copper/Nickel Metal-Organic Framework Nanosheets for Non-Enzymatic Electrochemical Glucose Detection.Micromachines (Basel). 2023 Sep 30;14(10):1896. doi: 10.3390/mi14101896. Micromachines (Basel). 2023. PMID: 37893332 Free PMC article.
-
Enzyme-Based Solid-Phase Electrochemiluminescence Sensors with Stable, Anchored Emitters for Sensitive Glucose Detection.Biosensors (Basel). 2025 May 21;15(5):332. doi: 10.3390/bios15050332. Biosensors (Basel). 2025. PMID: 40422071 Free PMC article.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. International Diabetes Federation; Brussels, Belgium: 2021. [(accessed on 16 April 2023)]. Available online: https://diabetesatlas.org/2022-reports/
-
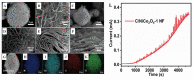
- Naderi L., Shahrokhian S., Amini M.K., Hafezi Kahnamouei M. Comparison of electrocatalytic performance of CuCo2O4 nanorods and nanospheres decorated with Co3S4 nanosheets for electrochemical sensing of hydrogen peroxide and glucose in human serum. ACS Appl. Nano Mater. 2023;6:2755–2769. doi: 10.1021/acsanm.2c05164. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

