Post-Polio Syndrome Revisited
- PMID: 37092507
- PMCID: PMC10123742
- DOI: 10.3390/neurolint15020035
Post-Polio Syndrome Revisited
Abstract
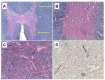
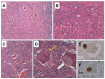
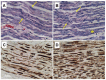
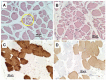
Post-polio syndrome (PPS) is characterized by recrudescence or worsening of motor neuron disease symptoms decades after recovery from acute paralytic poliovirus infection, i.e., poliomyelitis. PPS afflicts between 25% and 40% of poliomyelitis survivors and mimics motor neuron diseases (MNDs), such as amyotrophic lateral sclerosis (ALS), due to its selective impairment, degeneration, or death of motor neurons in the brainstem and spinal cord. Herein, we report a case of PPS in a 68-year-old man with a remote history of bulbar and cervical cord involvement by poliomyelitis, review the relevant literature, and contrast the salient histopathologic features that distinguish our case of PPS from ALS.
Keywords: amyotrophic lateral sclerosis; motor neuron disease; post-polio syndrome.
Conflict of interest statement
All authors declare to have no conflict of interest related to this manuscript.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

