Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010-2018
- PMID: 37092514
- PMCID: PMC10123615
- DOI: 10.3390/ijns9020020
Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010-2018
Abstract
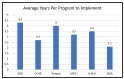
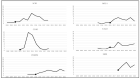
The Recommended Uniform Screening Panel (RUSP) is the list of conditions recommended by the US Secretary of Health and Human Services for inclusion in state newborn screening (NBS). During 2010-2022, seven conditions were added to the RUSP: severe combined immunodeficiency (SCID) (2010), critical congenital heart disease (CCHD) (2011), glycogen storage disease, type II (Pompe) (2015), mucopolysaccharidosis, type I (MPS I) (2016), X-linked adrenoleukodystrophy (X-ALD) (2016), spinal muscular atrophy (SMA) (2018), and mucopolysaccharidosis, type II (MPS II) (2022). The adoption of SCID and CCHD newborn screening by programs in all 50 states and three territories (Washington, D.C.; Guam; and Puerto Rico) took 8.6 and 6.8 years, respectively. As of December 2022, 37 programs screen for Pompe, 34 for MPS I, 32 for X-ALD, and 48 for SMA. The pace of implementation based on the average additional number of NBS programs per year was most rapid for SMA (11.3), followed by CCHD (7.8), SCID (6.2), MPS I (5.4), Pompe (4.9), and X-ALD (4.7).
Keywords: new conditions; newborn screening; public health.
Conflict of interest statement
The authors declare they have no conflict of competing interest.
Figures
References
-
- Watson M.S., Mann M.Y., Lloyd-Puryear M.A., Rinaldo P., Howell R.R. American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: Toward a uniform screening panel and system—Executive summary. Pediatrics. 2006;117((Suppl. S3)):S296–S307. doi: 10.1542/peds.2005-2633I. - DOI - PubMed
-
- Kemper A., Green N., Calonge N., Lam W.K.K., Comeau A., Goldenberg A., Ojodu J., Prosser L.A., Tanksley S., Bocchini J.A. Decision-making process for conditions nominated to the Recommended Uniform Screening Panel: Statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet. Med. 2014;16:183–187. doi: 10.1038/gim.2013.98. - DOI - PubMed
-
- Calonge N., Green N., Rinaldo P., Lloyd-Puryear M., Dougherty D., Boyle C., Watson M., Trotter T., Terry S., Howell R., et al. Committee report: Method for evaluating conditions nominated for population-based screening of newborns and children. Genet. Med. 2010;12:153–159. doi: 10.1097/GIM.0b013e3181d2af04. - DOI - PubMed
-
- Kemper A.R., Lam W.K.K., Ream M., Lennox A. NBS Implementation for Conditions Added to the RUSP; Proceedings of the ACHDNC Meeting; Rockville, MD, USA. 1 August 2019; [(accessed on 16 December 2022)]. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/herita....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

