Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network
- PMID: 37092521
- PMCID: PMC10123631
- DOI: 10.3390/hematolrep15020027
Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network
Abstract
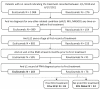
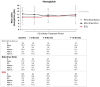
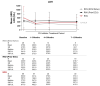
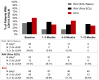
Paroxysmal nocturnal hemoglobinuria (PNH), a rare acquired hematologic disorder, can be treated with C5 inhibitors (C5i) such as eculizumab or ravulizumab. This retrospective study is the first to describe real-world treatment patterns and changes in hematologic PNH-monitoring laboratory tests among C5i-treated US patients. Data were extracted from TriNetX Dataworks Network and included patients with a PNH diagnosis between 1 January 2010, and 20 August 2021. Patients were stratified into three cohorts based on their C5i usage: eculizumab, ravulizumab (prior eculizumab), and ravulizumab (eculizumab naïve). Hematological markers (hemoglobin [Hb], lactate dehydrogenase [LDH], and absolute reticulocyte count [ARC]) and relevant clinical events (e.g., breakthrough hemolysis [BTH], complement-amplifying conditions [CAC], thrombosis, infection, and all-cause mortality) were captured any time within 12 months post-index treatment. Of the 143 (eculizumab), 43 (ravulizumab, prior eculizumab), and 33 (ravulizumab, eculizumab naïve) patients, mean age across cohorts was 42-51 years, 55-61% were female, 63-73% were White, and 33-40% had aplastic anemia. Among all cohorts 12 months post-C5i treatment, 50-82% remained anemic, 8-32% required ≥1 transfusion, and 13-59% had BTH, of which 33%-54% had CACs. Additionally, thrombosis was seen in 7-15% of patients, infection in 20-25%, and mortality in 1-7%. These findings suggest many C5i-treated patients experience suboptimal disease control.
Keywords: C5 inhibitors; anemia; breakthrough hemolysis; eculizumab; hematological outcome; hemoglobin; paroxysmal nocturnal hemoglobinuria; ravulizumab; transfusion.
Conflict of interest statement
JF and MMY are employees of Apellis Pharmaceuticals, Inc. and may own stock/stock options. KB is an employee of TriNetX, LLC., which has received research funding from Apellis Pharmaceuticals, Inc. SK and HC were employees of TriNetX, LLC. at the time the study was conducted.
Figures
Similar articles
-
Real-World Prevalence and Outcomes of Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors in the US: A Retrospective Claims Database Analysis.J Health Econ Outcomes Res. 2025 Aug 15;12(2):66-74. doi: 10.36469/001c.142049. eCollection 2025. J Health Econ Outcomes Res. 2025. PMID: 40827257 Free PMC article.
-
Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria.Haematologica. 2021 Jan 1;106(1):230-237. doi: 10.3324/haematol.2019.236877. Haematologica. 2021. PMID: 31949012 Free PMC article. Clinical Trial.
-
Cost burden of breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria receiving ravulizumab versus eculizumab.Hematology. 2020 Dec;25(1):327-334. doi: 10.1080/16078454.2020.1807226. Hematology. 2020. PMID: 32856539
-
Danicopan (Voydeya): Indication: As an add-on to ravulizumab or eculizumab for the treatment of adult patients with paroxysmal nocturnal hemoglobinuria who have residual hemolytic anemia due to extravascular hemolysis: Reimbursement Recommendation [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2024 Nov. Report No.: SR0815. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2024 Nov. Report No.: SR0815. PMID: 39693457 Free Books & Documents. Review.
-
Ravulizumab: a novel C5 inhibitor for the treatment of paroxysmal nocturnal hemoglobinuria.Ther Adv Hematol. 2019 Sep 10;10:2040620719874728. doi: 10.1177/2040620719874728. eCollection 2019. Ther Adv Hematol. 2019. PMID: 31534662 Free PMC article. Review.
Cited by
-
Effectiveness of Iptacopan Versus C5 Inhibitors in Complement Inhibitor-Naive Patients With Paroxysmal Nocturnal Haemoglobinuria.EJHaem. 2025 May 20;6(3):e270055. doi: 10.1002/jha2.70055. eCollection 2025 Jun. EJHaem. 2025. PMID: 40395624 Free PMC article.
-
C3d-Targeted factor H inhibits tissue complement in disease models and reduces glomerular injury without affecting circulating complement.Mol Ther. 2024 Apr 3;32(4):1061-1079. doi: 10.1016/j.ymthe.2024.02.001. Epub 2024 Feb 20. Mol Ther. 2024. PMID: 38382529 Free PMC article.
-
The cost-effectiveness of pegcetacoplan in complement treatment-naïve adults with paroxysmal nocturnal hemoglobinuria in the USA.J Comp Eff Res. 2023 Oct;12(10):e230055. doi: 10.57264/cer-2023-0055. Epub 2023 Sep 1. J Comp Eff Res. 2023. PMID: 37655691 Free PMC article.
-
Effect of Target-Mediated Disposition on Iptacopan Clinical Pharmacokinetics in Participants with Normal or Impaired Hepatic Function.Clin Pharmacol Ther. 2025 May;117(5):1358-1368. doi: 10.1002/cpt.3559. Epub 2025 Jan 24. Clin Pharmacol Ther. 2025. PMID: 39856537 Free PMC article. Clinical Trial.
-
Patient-reported improvements in paroxysmal nocturnal hemoglobinuria treated with iptacopan from 2 phase 3 studies.Blood Adv. 2025 Apr 22;9(8):1816-1826. doi: 10.1182/bloodadvances.2024014652. Blood Adv. 2025. PMID: 39774762 Free PMC article. Clinical Trial.
References
-
- Schrezenmeier H., Muus P., Socié G., Szer J., Urbano-Ispizua A., Maciejewski J.P., Brodsky R.A., Bessler M., Kanakura Y., Rosse W., et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014;99:922–929. doi: 10.3324/haematol.2013.093161. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

