Clinical and functional heterogeneity associated with the disruption of retinoic acid receptor beta
- PMID: 37092537
- PMCID: PMC10757562
- DOI: 10.1016/j.gim.2023.100856
Clinical and functional heterogeneity associated with the disruption of retinoic acid receptor beta
Abstract
Purpose: Dominant variants in the retinoic acid receptor beta (RARB) gene underlie a syndromic form of microphthalmia, known as MCOPS12, which is associated with other birth anomalies and global developmental delay with spasticity and/or dystonia. Here, we report 25 affected individuals with 17 novel pathogenic or likely pathogenic variants in RARB. This study aims to characterize the functional impact of these variants and describe the clinical spectrum of MCOPS12.
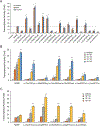
Methods: We used in vitro transcriptional assays and in silico structural analysis to assess the functional relevance of RARB variants in affecting the normal response to retinoids.
Results: We found that all RARB variants tested in our assays exhibited either a gain-of-function or a loss-of-function activity. Loss-of-function variants disrupted RARB function through a dominant-negative effect, possibly by disrupting ligand binding and/or coactivators' recruitment. By reviewing clinical data from 52 affected individuals, we found that disruption of RARB is associated with a more variable phenotype than initially suspected, with the absence in some individuals of cardinal features of MCOPS12, such as developmental eye anomaly or motor impairment.
Conclusion: Our study indicates that pathogenic variants in RARB are functionally heterogeneous and associated with extensive clinical heterogeneity.
Keywords: Dystonia; Global developmental delay; Microphthalmia; Retinoic acid; Retinoic acid receptor beta.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest J.R.L. owns stock in 23andMe and is a paid consultant for Genome International. All other authors declare no conflicts of interest.
Figures
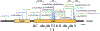

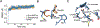
References
-
- Srour M, Caron V, Pearson T, et al. Gain-of-Function Mutations in RARB Cause Intellectual Disability with Progressive Motor Impairment. Hum Mutat. 2016;37(8):786–793. - PubMed
-
- MJ A. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

