β-Catenin signaling in alveolar macrophages enhances lung metastasis through a TNF-dependent mechanism
- PMID: 37092550
- PMCID: PMC10243816
- DOI: 10.1172/jci.insight.160978
β-Catenin signaling in alveolar macrophages enhances lung metastasis through a TNF-dependent mechanism
Abstract
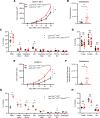
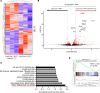
The main cause of malignancy-related mortality is metastasis. Although metastatic progression is driven by diverse tumor-intrinsic mechanisms, there is a growing appreciation for the contribution of tumor-extrinsic elements of the tumor microenvironment, especially macrophages, which correlate with poor clinical outcomes. Macrophages consist of bone marrow-derived and tissue-resident populations. In contrast to bone marrow-derived macrophages, the transcriptional pathways that govern the pro-metastatic activities of tissue-resident macrophages (TRMs) remain less clear. Alveolar macrophages (AMs) are a TRM population with critical roles in tissue homeostasis and metastasis. Wnt/β-catenin signaling is a hallmark of cancer and has been identified as a pathologic regulator of AMs in infection. We tested the hypothesis that β-catenin expression in AMs enhances metastasis in solid tumor models. Using a genetic β-catenin gain-of-function approach, we demonstrated that (a) enhanced β-catenin in AMs heightened lung metastasis; (b) β-catenin activity in AMs drove a dysregulated inflammatory program strongly associated with Tnf expression; and (c) localized TNF-α blockade abrogated this metastatic outcome. Last, β-catenin gene CTNNB1 and TNF expression levels were positively correlated in AMs of patients with lung cancer. Overall, our findings revealed a Wnt/β-catenin/TNF-α pro-metastatic axis in AMs with potential therapeutic implications against tumors refractory to the antineoplastic actions of TNF-α.
Keywords: Breast cancer; Macrophages; Mouse models; Oncology.
Conflict of interest statement
Figures




References
-
- Stathis A, Bertoni F. BET proteins as targets for anticancer treatment. Cancer Discov. 2018;8(1):24–36. doi: 10.1158/2159-8290.CD-17-0605. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

