Lymph Node Follicle-Targeting STING Agonist Nanoshells Enable Single-Shot M2e Vaccination for Broad and Durable Influenza Protection
- PMID: 37092580
- PMCID: PMC10265066
- DOI: 10.1002/advs.202206521
Lymph Node Follicle-Targeting STING Agonist Nanoshells Enable Single-Shot M2e Vaccination for Broad and Durable Influenza Protection
Abstract
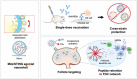
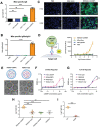
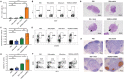
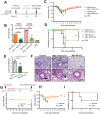
The highly conserved matrix protein 2 ectodomain (M2e) of influenza viruses presents a compelling vaccine antigen candidate for stemming the pandemic threat of the mutation-prone pathogen, yet the low immunogenicity of the diminutive M2e peptide renders vaccine development challenging. A highly potent M2e nanoshell vaccine that confers broad and durable influenza protectivity under a single vaccination is shown. Prepared via asymmetric ionic stabilization for nanoscopic curvature formation, polymeric nanoshells co-encapsulating high densities of M2e peptides and stimulator of interferon genes (STING) agonists are prepared. Robust and long-lasting protectivity against heterotypic influenza viruses is achieved with a single administration of the M2e nanoshells in mice. Mechanistically, molecular adjuvancy by the STING agonist and nanoshell-mediated prolongation of M2e antigen exposure in the lymph node follicles synergistically contribute to the heightened anti-M2e humoral responses. STING agonist-triggered T cell helper functions and extended residence of M2e peptides in the follicular dendritic cell network provide a favorable microenvironment that induces Th1-biased antibody production against the diminutive antigen. These findings highlight a versatile nanoparticulate design that leverages innate immune pathways for enhancing the immunogenicity of weak immunogens. The single-shot nanovaccine further provides a translationally viable platform for pandemic preparedness.
Keywords: follicular dendritic cells; germinal center; lymph node follicle targeting; matrix protein 2 ectodomain antigen; nanoshell; stimulator of interferon genes agonist; universal influenza vaccine.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

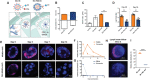
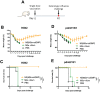
References
-
- Subbarao K., Murphy B. R., Fauci A. S., Immunity 2006, 24, 5. - PubMed
-
- Belongia E. A., Kieke B. A., Donahue J. G., Greenlee R. T., Balish A., Foust A., Lindstrom S., Shay D. K., J. Infect. Dis. 2009, 199, 159. - PubMed
-
- a) Nachbagauer R., Feser J., Naficy A., Bernstein D. I., Guptill J., Walter E. B., Berlanda‐Scorza F., Stadlbauer D., Wilson P. C., Aydillo T., Behzadi M. A., Bhavsar D., Bliss C., Capuano C., Carreno J. M., Chromikova V., Claeys C., Coughlan L., Freyn A. W., Gast C., Javier A., Jiang K., Mariottini C., McMahon M., McNeal M., Solorzano A., Strohmeier S., Sun W., Van der Wielen M., Innis B. L., et al., Nat. Med. 2021, 27, 106; - PubMed
- b) Darricarrere N., Qiu Y., Kanekiyo M., Creanga A., Gillespie R. A., Moin S. M., Saleh J., Sancho J., Chou T. H., Zhou Y. F., Zhang R. J., Dai S. J., Moody A., Saunders K. O., Crank M. C., Mascola J. R., Graham B. S., Wei C. J., Nabel G. J., Sci. Transl. Med. 2021, 13, eabe5449; - PubMed
- c) Tseng Y. C., Wu C. Y., Liu M. L., Chen T. H., Chiang W. L., Yu Y. H., Jan J. T., Lin K. I., Wong C. H., Ma C., Proc. Natl. Acad. Sci. USA 2019, 116, 4200; - PMC - PubMed
- d) Laursen N. S., Friesen R. H. E., Zhu X., Jongeneelen M., Blokland S., Vermond J., van Eijgen A., Tang C., van Diepen H., Obmolova G., van der Neut Kolfschoten M., Zuijdgeest D., Straetemans R., Hoffman R. M. B., Nieusma T., Pallesen J., Turner H. L., Bernard S. M., Ward A. B., Luo J., Poon L. L. M., Tretiakova A. P., Wilson J. M., Limberis M. P., Vogels R., Brandenburg B., Kolkman J. A., Wilson I. A., Science 2018, 362, 598; - PMC - PubMed
- e) Lin P. H., Liang C. Y., Yao B. Y., Chen H. W., Pan C. F., Wu L. L., Lin Y. H., Hsu Y. S., Liu Y. H., Chen P. J., Hu C. J., Yang H. C., Mol. Ther. Methods Clin. Dev. 2021, 21, 299; - PMC - PubMed
- f) Boyoglu‐Barnum S., Ellis D., Gillespie R. A., Hutchinson G. B., Park Y. J., Moin S. M., Acton O. J., Ravichandran R., Murphy M., Pettie D., Matheson N., Carter L., Creanga A., Watson M. J., Kephart S., Ataca S., Vaile J. R., Ueda G., Crank M. C., Stewart L., Lee K. K., Guttman M., Baker D., Mascola J. R., Veesler D., Graham B. S., King N. P., Kanekiyo M., Nature 2021, 592, 623. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
