Adjunctive inhaled amikacin in infants with Ventilator-Associated Pneumonia optimizes the complex antimicrobial therapy: pilot study
- PMID: 37092633
- PMCID: PMC10210574
- DOI: 10.23750/abm.v94i2.13910
Adjunctive inhaled amikacin in infants with Ventilator-Associated Pneumonia optimizes the complex antimicrobial therapy: pilot study
Abstract
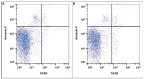
Background and aim: VAP remains the second leading cause of death among the patients with nosocomial infections and its incidence varies significantly from 5% to 60% reaching on average 10 %. It is of crucial importance to develop novel treatment approaches and optimize the existing ones. Thus, the aim of this pilot study was to study the laboratory-microbiological effect of inhaled aminoglycosides in a complex treatment of patients with ventilator-associatedpneumonia(VAP). Methods: To study the laboratory-microbiological effect of adjunctive inhaled aminoglycosides in the treatment of VAP, twenty enrolled patients were randomly subdivided into 2 groups (n=10). Amikacin was administered via a nebulizer starting from the first day of VAP manifestation. Inhalations were performed BID for 7 days via a nebulizer integrated into the breathing circuit. We assessed: cell membrane alterations in leukocytes, Annexin V/7-AAD staining for leukocytes, ROS detection assay for leukocytes.
Results: Adjunctive administration of inhaled amikacin reduced the fluorescence intensity ratio more efficiently compared with the intravenous antimicrobial treatment with no aerosolized amikacin following both 48 h and 96 h of treatment. The amount of dead necrotic annexin V-negative, 7-AAD-positive leukocytes was significantly lower under the use of inhaled amikacin than at the beginning of treatment. Conclusions In this pilot study, we found that administration of aerosolized amikacin combined with the systemic antimicrobial therapy improves the clinical outcome of patients with VAP, effective early microbial decrease in the sputum, reduces reactive oxygen species generation in leukocytes and the degree of leukocyte apoptosis and necrosis, decreases VAP-mediated cell membrane alterations of circulating leukocytes.
Conflict of interest statement
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Figures



Similar articles
-
Use of aerosolized aminoglycosides in the treatment of Gram-negative ventilator-associated pneumonia.Surg Infect (Larchmt). 2007 Jun;8(3):349-57. doi: 10.1089/sur.2006.041. Surg Infect (Larchmt). 2007. PMID: 17635058
-
Aerosolized Amikacin as Adjunctive Therapy of Ventilator-associated Pneumonia Caused by Multidrug-resistant Gram-negative Bacteria: A Single-center Randomized Controlled Trial.Chin Med J (Engl). 2017 May 20;130(10):1196-1201. doi: 10.4103/0366-6999.205846. Chin Med J (Engl). 2017. PMID: 28485320 Free PMC article. Clinical Trial.
-
Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: a prospective study.Respir Med. 2008 Mar;102(3):407-12. doi: 10.1016/j.rmed.2007.10.011. Epub 2007 Dec 3. Respir Med. 2008. PMID: 18060758
-
Novel amikacin inhaled formulation for the treatment of lower respiratory tract infections.Drugs Today (Barc). 2013 Nov;49(11):683-92. doi: 10.1358/dot.2013.49.11.2033101. Drugs Today (Barc). 2013. PMID: 24308015 Review.
-
Why don't we have more inhaled antibiotics to treat ventilator-associated pneumonia?Clin Microbiol Infect. 2019 Oct;25(10):1195-1199. doi: 10.1016/j.cmi.2019.04.018. Epub 2019 Apr 26. Clin Microbiol Infect. 2019. PMID: 31035015 Review.
Cited by
-
Use of mucolytics and inhaled antibiotics in the NICU.J Perinatol. 2025 Jan;45(1):5-12. doi: 10.1038/s41372-024-02178-w. Epub 2024 Nov 19. J Perinatol. 2025. PMID: 39562833 Free PMC article. Review.
-
Nebulized Antibiotics for Preventing and Treating Gram-Negative Respiratory Infections in Critically Ill Patients: An Overview of Reviews.Antibiotics (Basel). 2025 Apr 2;14(4):370. doi: 10.3390/antibiotics14040370. Antibiotics (Basel). 2025. PMID: 40298497 Free PMC article. Review.
References
-
- Кohbodi GA, Rajasurya V, Noor A. Ventilator-associated Pneumonia 2021 Aug 22. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022 Jan. PMID: 29939533. - PubMed
-
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63(5):e61–e111. doi: 10.1093/cid/ciw353. Epub 2016 Jul 14. Erratum in: Clin Infect Dis. 2017 May 1;64(9):1298. Erratum in: Clin Infect Dis. 2017 Oct 15;65(8):1435. Erratum in: Clin Infect Dis. 2017 Nov 29;65(12):2161. - PMC - PubMed
-
- Metersky ML, Wang Y, Klompas M, et al. Trend in Ventilator-Associated Pneumonia Rates Between 2005 and 2013. JAMA. 2016 Dec 13;316(22):2427–2429. doi: 10.1001/jama.2016.16226. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

