Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells
- PMID: 37092860
- PMCID: PMC10195137
- DOI: 10.3724/abbs.2023068
Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells
Abstract
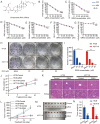
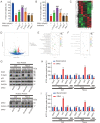
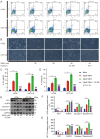
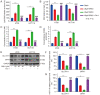
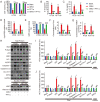
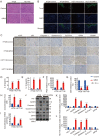
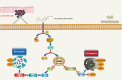
Ginsenoside Rh3 (GRh3) is a seminatural product obtained by chemical processing after isolation from Chinese herbal medicine that has strong antitumor activity against human tumors. However, its antitumor role remains to be elucidated. The aim of this study is to explore the mechanisms underlying the tumor suppressive activity of GRh3 from the perspective of pyroptosis and ferroptosis. GRh3 eliminates colorectal cancer (CRC) cells by activating gasdermin D (GSDMD)-dependent pyroptosis and suppressing solute carrier family 7 member 11 (SLC7A11), resulting in ferroptosis activation through the Stat3/p53/NRF2 axis. GRh3 suppresses nuclear factor erythroid 2-related factor 2 (NRF2) entry into the nucleus, leading to the decrease of heme oxygenase 1 (HO-1) expression, which in turn promotes NOD-like receptor thermal protein domain associated protein 3 (NLRP3) and caspase-1 expression. Finally, caspase-1 activates GSDMD-dependent pyroptosis. Furthermore, GRh3 prevents NRF2 from entering the nucleus, which suppresses SLC7A11, causing the depletion of glutathione (GSH) and accumulation of iron, lipid reactive oxygen species (ROS) and malondialdehyde (MDA), and eventually leading to ferroptosis in CRC cells. In addition, GRh3 effectively inhibits the proliferation of CRC cells in vitro and in nude mouse models. Collectively, GRh3 triggers pyroptotic cell death and ferroptotic cell death in CRC cells via the Stat3/p53/NRF2 axis with minimal harm to normal cells, showing great anticancer potential.
Keywords: Stat3/p53/NRF2 axis; colorectal cancer; ferroptosis; ginsenoside Rh3; pyroptosis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. . 2021;71:209–249. doi: 10.3322/caac.21660. - DOI - PubMed
-
- Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. . 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. - DOI - PMC - PubMed
-
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, et al. NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Comprehensive Cancer Network. . 2020;18:806–815. doi: 10.6004/jnccn.2020.0032. - DOI - PubMed
-
- Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. . 2020;21:678–695. doi: 10.1038/s41580-020-0270-8. - DOI - PubMed
-
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1 . Proc Natl Acad Sci USA. . 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

