The Novel DNA Binding Mechanism of Ridinilazole, a Precision Clostridiodes difficile Antibiotic
- PMID: 37093023
- PMCID: PMC10246881
- DOI: 10.1128/aac.01563-22
The Novel DNA Binding Mechanism of Ridinilazole, a Precision Clostridiodes difficile Antibiotic
Abstract
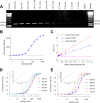
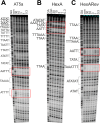
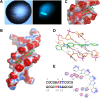
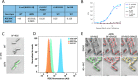
Clostridioides difficile infection (CDI) causes substantial morbidity and mortality worldwide with limited antibiotic treatment options. Ridinilazole is a precision bisbenzimidazole antibiotic being developed to treat CDI and reduce unacceptably high rates of infection recurrence in patients. Although in late clinical development, the precise mechanism of action by which ridinilazole elicits its bactericidal activity has remained elusive. Here, we present conclusive biochemical and structural data to demonstrate that ridinilazole has a primary DNA binding mechanism, with a co-complex structure confirming binding to the DNA minor groove. Additional RNA-seq data indicated early pleiotropic changes to transcription, with broad effects on multiple C. difficile compartments and significant effects on energy generation pathways particularly. DNA binding and genomic localization was confirmed through confocal microscopy utilizing the intrinsic fluorescence of ridinilazole upon DNA binding. As such, ridinilazole has the potential to be the first antibiotic approved with a DNA minor groove binding mechanism of action.
Keywords: Clostridium difficile; antibiotic; mechanisms of action.
Conflict of interest statement
The authors declare a conflict of interest. C.S.M., T.A., C.C., E.D., N.N., M.M., and D.J.P. are employees of and/or hold stock/stock options in Summit Therapeutics. Work contributed by K.R.F. was funded through a research collaboration agreement between University of Southampton and Summit Therapeutics. Work contributed by S. M. and S. R. was funded through a research collaboration agreement between Domainex Ltd and Summit Therapeutics. Work contributed by C.H., K.B., K.G., M.J.A., E.B., and K.W.G. was funded through a research collaboration agreement between University of Houston and Summit Therapeutics. K.W.G. has additional research funding paid to his university from Acurx Pharmaceuticals, Paratek Pharmaceuticals, and Seres Health.
Figures


References
-
- CDC. 2019. Antibiotic resistance threats in the United States, 2019. CDC, Atlanta, GA.
-
- Enoch DA, Murray-Thomas T, Adomakoh N, Dedman D, Georgopali A, Francis NA, Karas A. 2020. Risk of complications and mortality following recurrent and non-recurrent Clostridioides difficile infection: a retrospective observational database study in England. J Hosp Infect 106:793–803. doi:10.1016/j.jhin.2020.09.025. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

