Heterogeneity and Recombination of Foot-and-Mouth Disease Virus during Multi-Strain Coinfection of Cattle
- PMID: 37093054
- PMCID: PMC10286704
- DOI: 10.1128/msphere.00643-22
Heterogeneity and Recombination of Foot-and-Mouth Disease Virus during Multi-Strain Coinfection of Cattle
Abstract
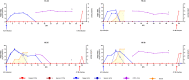
Superinfection of cattle persistently infected with foot-and-mouth disease virus (FMDV), with a heterologous FMDV strain has been shown to generate novel recombinant viruses. In this study, we investigated the pathogenesis events within specific tissues associated with FMDV coinfections in cattle subjected to either simultaneous or serial exposure to two distinct strains of FMDV. Both strains of FMDV (one each of serotypes O and A) were similarly localized to the nasopharyngeal mucosa during the early stages of infection. However, while no recombinant FMDV genomes were recovered from simultaneously coinfected cattle, interserotypic recombinants were isolated from nasopharyngeal tissue samples obtained at 48 h after heterologous superinfection of a persistently infected FMDV carrier. Additionally, analysis of FMDV genomes obtained from replicate nasopharyngeal tissue samples demonstrated that adjacent segments of the mucosa were sometimes infected by distinct viruses, demonstrating a multifocal and heterogeneous distribution of FMDV infection during primary and persistent phases of infection. This work indicates that superinfection of FMDV carriers may be an important source of emergent recombinant strains of FMDV in areas where multiple strains are co-circulating. IMPORTANCE Foot-and-mouth disease (FMD) is a socioeconomically impactful livestock disease with a complex epidemiology and ecology. Although recombinant viruses have been identified in field samples, the mechanisms of emergence of those viruses have never been elucidated. This current study demonstrates how serial infection of cattle with two distinct serotypes of FMD virus (FMDV) leads to rapid generation of recombinant viruses in the upper respiratory tracts of infected animals. This finding is particularly relevant in relation to the management of persistently infected FMDV carrier cattle that can maintain subclinical FMDV infection for months to years after an initial infection. Such carrier animals may function as mixing vessels that facilitate the emergence of novel recombinant FMDV strains in areas where multiple virus strains are in circulation.
Keywords: FMD; FMDV; cattle; foot-and-mouth disease; foot-and-mouth disease virus; infectious disease; pathogenesis; recombination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Office International des Epizooties (OIE). 2022. Application for official recognition by the OI of free status for foot-and-mouth disease virus. In Terrestrial Animal Health Code. Available from https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/current/ch.... WOAH, Paris, France.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
