Capsulized Fecal Microbiota Transplantation Induces Remission in Patients with Ulcerative Colitis by Gut Microbial Colonization and Metabolite Regulation
- PMID: 37093057
- PMCID: PMC10269780
- DOI: 10.1128/spectrum.04152-22
Capsulized Fecal Microbiota Transplantation Induces Remission in Patients with Ulcerative Colitis by Gut Microbial Colonization and Metabolite Regulation
Abstract
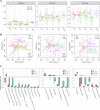
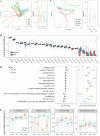
Fecal microbiota transplantation (FMT) can induce clinical remission in ulcerative colitis (UC) patients. Enemas, nasoduodenal tubes, and colonoscopies are the most common routes for FMT administration. However, there is a lack of definitive evidence regarding the effectiveness of capsulized FMT treatment in UC patients. In this study, we administered capsulized FMT to 22 patients with active UC to assess the efficiency of capsulized FMT and determine the specific bacteria and metabolite factors associated with the response to clinical remission. Our results showed that the use of capsulized FMT was successful in the treatment of UC patients. Capsulized FMT induced clinical remission and clinical response in 57.1% (12 of 21) and 76.2% (16 of 21) of UC patients, respectively. Gut bacterial richness was increased after FMT in patients who achieved remission. Patients in remission after FMT exhibited enrichment of Alistipes sp. and Odoribacter splanchnicus, along with increased levels of indolelactic acid. Patients who did not achieve remission exhibited enrichment of Escherichia coli and Klebsiella and increased levels of biosynthesis of 12,13-DiHOME (12,13-dihydroxy-9Z-octadecenoic acid) and lipopolysaccharides. Furthermore, we identified a relationship between specific bacteria and metabolites and the induction of remission in patients. These findings may provide new insights into FMT in UC treatment and provide reference information about therapeutic microbial manipulation of FMT to enhance its effects. (This study has been registered at ClinicalTrails.gov under registration no. NCT03426683). IMPORTANCE Fecal microbiota transplantation has been successfully used in patients. Recently, capsulized FMT was reported to induce a response in patients with UC. However, limited patients were enrolled in such studies, and the functional factors of capsulized FMT have not been reported in the remission of patients with UC. In this study, we prospectively recruited patients with UC to receive capsulized FMT. First, we found that capsulized FMT could induce clinical remission in 57.1% of patients and clinical response in 76.2% after 12 weeks, which was more acceptable. Second, we found a relationship between the decrease of opportunistic pathogen and lipopolysaccharide synthesis in patients in remission after capsulized FMT. We also identified an association between specific bacteria and metabolites and remission induction in patients after capsulized FMT. These findings put forward a possibility for patients to receive FMT at home and provide reference information about therapeutic microbial manipulation of FMT to enhance its effects.
Keywords: capsules; fecal microbiota transplantation; gut microbiome; metabolism; microbiome; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368:407–415. doi: 10.1056/NEJMoa1205037. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

