Zika virus-induces metabolic alterations in fetal neuronal progenitors that could influence in neurodevelopment during early pregnancy
- PMID: 37093064
- PMCID: PMC10151830
- DOI: 10.1242/bio.059889
Zika virus-induces metabolic alterations in fetal neuronal progenitors that could influence in neurodevelopment during early pregnancy
Abstract
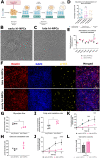
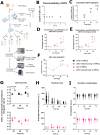
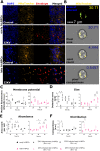
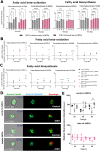
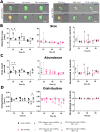
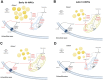
Cortical development consists of an orchestrated process in which progenitor cells exhibit distinct fate restrictions regulated by time-dependent activation of energetic pathways. Thus, the hijacking of cellular metabolism by Zika virus (ZIKV) to support its replication may contribute to damage in the developing fetal brain. Here, we showed that ZIKV replicates differently in two glycolytically distinct pools of cortical progenitors derived from human induced pluripotent stem cells (hiPSCs), which resemble the metabolic patterns of quiescence (early hi-NPCs) and immature brain cells (late hi-NPCs) in the forebrain. This differential replication alters the transcription of metabolic genes in both pools of cortical progenitors but solely upregulates the glycolytic capacity of early hi-NPCs. Analysis using Imagestream® revealed that, during early stages of ZIKV replication, in early hi-NPCs there is an increase in lipid droplet abundance and size. This stage of ZIKV replication significantly reduced the mitochondrial distribution in both early and late hi-NPCs. During later stages of ZIKV replication, late hi-NPCs show reduced mitochondrial size and abundance. The finding that there are alterations of cellular metabolism during ZIKV infection which are specific to pools of cortical progenitors at different stages of maturation may help to explain the differences in brain damage over each trimester.
Keywords: Fetal neurodevelopment; Metabolism; Neurometabolism; Neuronal progenitors; ZIKV; Zika virus.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Anfasa, F., Goeijenbier, M., Widagdo, W., Siegers, J. Y., Mumtaz, N., Okba, N., van Riel, D., Rockx, B., Koopmans, M. P. G., Meijers, J. C. M.et al. (2019). Zika virus infection induces elevation of tissue factor production and apoptosis on human umbilical vein endothelial cells. Front. Microbiol. 10, 817. 10.3389/fmicb.2019.00817 - DOI - PMC - PubMed
-
- Aubry, F., Jacobs, S., Darmuzey, M., Lequime, S., Delang, L., Fontaine, A., Jupatanakul, N., Miot, E. F., Dabo, S., Manet, C.et al. (2021). Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat. Commun. 12, 1-14. 10.1038/s41467-021-21199-z - DOI - PMC - PubMed
-
- Barbeito-Andrés, J., Pezzuto, P., Higa, L. M., Dias, A. A., Vasconcelos, J. M., Santos, T. M. P., Ferreira, J. C. C. G., Ferreira, R. O., Dutra, F. F., Rossi, A. D.et al. (2020). Congenital Zika syndrome is associated with maternal protein malnutrition. Sci. Adv. 6, eaaw6284. 10.1126/sciadv.aaw6284 - DOI - PMC - PubMed
-
- Beers, J., Gulbranson, D. R., George, N., Siniscalchi, L. I., Jones, J., Thomson, J. A. and Chen, G. (2012). Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 7, 2029-2040. 10.1038/nprot.2012.130 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

