Effects of Plasma-Derived Exosomal miRNA-19b-3p on Treg/T Helper 17 Cell Imbalance in Behçet's Uveitis
- PMID: 37093132
- PMCID: PMC10148662
- DOI: 10.1167/iovs.64.4.28
Effects of Plasma-Derived Exosomal miRNA-19b-3p on Treg/T Helper 17 Cell Imbalance in Behçet's Uveitis
Abstract
Purpose: To explore the potential role of plasma-derived exosomal microRNAs (miRNAs) in the development of regulatory T cell (Treg)/T helper 17 (Th17) cell imbalances in Behçet's uveitis (BU).
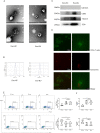
Methods: The exosome treatment was conducted to evaluate the effects of plasma exosomes from patients with active BU and healthy controls on the Treg/Th17 cell balance. miRNA sequencing analysis of plasma exosomes was conducted to identify differentially expressed miRNAs between patients with active BU and healthy controls. miRTarBase analysis and dual-luciferase reporter assays were conducted to identify the target genes of miR-19b-3p. CD4+T cells were transfected with miR-19b-3p mimic or inhibitor to evaluate its regulation of the Treg/Th17 cell balance. The Treg/Th17 cell balance in CD4+T cells was evaluated by flow cytometry and enzyme-linked immunosorbent assay.
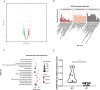
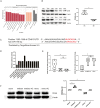
Results: Exosomes from patients with active BU promoted Th17 cell differentiation and inhibited Treg cell differentiation. MiRNA sequencing analysis revealed 177 upregulated and 274 downregulated miRNAs in plasma exosomes of patients with active BU. Among them, miR-19b-3p was significantly elevated, and its target genes were identified as being involved in T-cell differentiation. miR-19b-3p overexpression downregulated CD46 expression and the Treg/Th17 cell ratio in CD4+T cells from healthy controls, whereas miR-19b-3p inhibition reversed these regulatory effects and restored the Treg/Th17 cell balance of CD4+T cells from patients with active BU.
Conclusions: Plasma-derived exosomes from patients with active BU showed a markedly differential miRNA expression in comparison to healthy controls. Highly expressed miRNA-19b-3p could induce a Treg/Th17 cell imbalance, probably by downregulating CD46 expression.
Conflict of interest statement
Disclosure:
Figures

References
-
- Deuter CM, Kötter I, Wallace GR, Murray PI, Stübiger N, Zierhut M.. Behçet's disease: Ocular effects and treatment. Prog Retin Eye Res. 2008; 27(1): 111–136. - PubMed
-
- Mendes D, Correia M, Barbedo M, et al. .. Behçet's disease – a contemporary review. J Autoimmun. 2009; 32: 178–188. - PubMed
-
- Cunningham ET Jr, Tugal-Tutkun I, Khairallah M, Okada AA, Bodaghi B, Zierhut M. Behçet uveitis. Ocul Immunol Inflamm. 2017; 25(1): 2–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

