Neutralizing Monoclonal Antibody Use and COVID-19 Infection Outcomes
- PMID: 37093599
- PMCID: PMC10126875
- DOI: 10.1001/jamanetworkopen.2023.9694
Neutralizing Monoclonal Antibody Use and COVID-19 Infection Outcomes
Abstract
Importance: Evidence on the effectiveness and safety of COVID-19 therapies across a diverse population with varied risk factors is needed to inform clinical practice.
Objective: To assess the safety of neutralizing monoclonal antibodies (nMAbs) for the treatment of COVID-19 and their association with adverse outcomes.
Design, setting, and participants: This retrospective cohort study included 167 183 patients from a consortium of 4 health care systems based in California, Minnesota, Texas, and Utah. The study included nonhospitalized patients 12 years and older with a positive COVID-19 laboratory test collected between November 9, 2020, and January 31, 2022, who met at least 1 emergency use authorization criterion for risk of a poor outcome.
Exposure: Four nMAb products (bamlanivimab, bamlanivimab-etesevimab, casirivimab-imdevimab, and sotrovimab) administered in the outpatient setting.
Main outcomes and measures: Clinical and SARS-CoV-2 genomic sequence data and propensity-adjusted marginal structural models were used to assess the association between treatment with nMAbs and 4 outcomes: all-cause emergency department (ED) visits, hospitalization, death, and a composite of hospitalization or death within 14 days and 30 days of the index date (defined as the date of the first positive COVID-19 test or the date of referral). Patient index dates were categorized into 4 variant epochs: pre-Delta (November 9, 2020, to June 30, 2021), Delta (July 1 to November 30, 2021), Delta and Omicron BA.1 (December 1 to 31, 2021), and Omicron BA.1 (January 1 to 31, 2022).
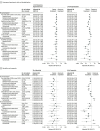
Results: Among 167 183 patients, the mean (SD) age was 47.0 (18.5) years; 95 669 patients (57.2%) were female at birth, 139 379 (83.4%) were White, and 138 900 (83.1%) were non-Hispanic. A total of 25 241 patients received treatment with nMAbs. Treatment with nMAbs was associated with lower odds of ED visits within 14 days (odds ratio [OR], 0.76; 95% CI, 0.68-0.85), hospitalization within 14 days (OR, 0.52; 95% CI, 0.45-0.59), and death within 30 days (OR, 0.14; 95% CI, 0.10-0.20). The association between nMAbs and reduced risk of hospitalization was stronger in unvaccinated patients (14-day hospitalization: OR, 0.51; 95% CI, 0.44-0.59), and the associations with hospitalization and death were stronger in immunocompromised patients (hospitalization within 14 days: OR, 0.31 [95% CI, 0.24-0.41]; death within 30 days: OR, 0.13 [95% CI, 0.06-0.27]). The strength of associations of nMAbs increased incrementally among patients with a greater probability of poor outcomes; for example, the ORs for hospitalization within 14 days were 0.58 (95% CI, 0.48-0.72) among those in the third (moderate) risk stratum and 0.41 (95% CI, 0.32-0.53) among those in the fifth (highest) risk stratum. The association of nMAb treatment with reduced risk of hospitalizations within 14 days was strongest during the Delta variant epoch (OR, 0.37; 95% CI, 0.31-0.43) but not during the Omicron BA.1 epoch (OR, 1.29; 95% CI, 0.68-2.47). These findings were corroborated in the subset of patients with viral genomic data. Treatment with nMAbs was associated with a significant mortality benefit in all variant epochs (pre-Delta: OR, 0.16 [95% CI, 0.08-0.33]; Delta: OR, 0.14 [95% CI, 0.09-0.22]; Delta and Omicron BA.1: OR, 0.10 [95% CI, 0.03-0.35]; and Omicron BA.1: OR, 0.13 [95% CI, 0.02-0.93]). Potential adverse drug events were identified in 38 treated patients (0.2%).
Conclusions and relevance: In this study, nMAb treatment for COVID-19 was safe and associated with reductions in ED visits, hospitalization, and death, although it was not associated with reduced risk of hospitalization during the Omicron BA.1 epoch. These findings suggest that targeted risk stratification strategies may help optimize future nMAb treatment decisions.
Conflict of interest statement
Figures


Comment in
-
Monoclonal Antibodies for the Treatment of COVID-19-Every Day You Fight Like You're Running Out of Time.JAMA Netw Open. 2023 Apr 3;6(4):e239702. doi: 10.1001/jamanetworkopen.2023.9702. JAMA Netw Open. 2023. PMID: 37093604 No abstract available.
References
-
- US Food & Drug Administration . Real-world evidence. US Food & Drug Administration. Updated January 31, 2023. Accessed March 2, 2022. https://www.fda.gov/science-research/science-and-research-special-topics...
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

