Precision medicine in complex diseases-Molecular subgrouping for improved prediction and treatment stratification
- PMID: 37093654
- PMCID: PMC10523928
- DOI: 10.1111/joim.13640
Precision medicine in complex diseases-Molecular subgrouping for improved prediction and treatment stratification
Abstract
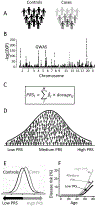
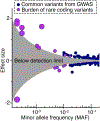
Complex diseases are caused by a combination of genetic, lifestyle, and environmental factors and comprise common noncommunicable diseases, including allergies, cardiovascular disease, and psychiatric and metabolic disorders. More than 25% of Europeans suffer from a complex disease, and together these diseases account for 70% of all deaths. The use of genomic, molecular, or imaging data to develop accurate diagnostic tools for treatment recommendations and preventive strategies, and for disease prognosis and prediction, is an important step toward precision medicine. However, for complex diseases, precision medicine is associated with several challenges. There is a significant heterogeneity between patients of a specific disease-both with regards to symptoms and underlying causal mechanisms-and the number of underlying genetic and nongenetic risk factors is often high. Here, we summarize precision medicine approaches for complex diseases and highlight the current breakthroughs as well as the challenges. We conclude that genomic-based precision medicine has been used mainly for patients with highly penetrant monogenic disease forms, such as cardiomyopathies. However, for most complex diseases-including psychiatric disorders and allergies-available polygenic risk scores are more probabilistic than deterministic and have not yet been validated for clinical utility. However, subclassifying patients of a specific disease into discrete homogenous subtypes based on molecular or phenotypic data is a promising strategy for improving diagnosis, prediction, treatment, prevention, and prognosis. The availability of high-throughput molecular technologies, together with large collections of health data and novel data-driven approaches, offers promise toward improved individual health through precision medicine.
Keywords: GWAS; complex diseases; genetic variations; genomic medicine; molecular profiling; multi omics; polygenic risk score (PRS); precision medicine.
© 2023 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
Conflicts of Interests
OAA is a consultant to
EM has received consultant honoraria from ALK, AstraZeneca, Chiesi, Sanofi and Viatris outside the submitted work.
SB has ownerships in Intomics A/S, Hoba Therapeutics Aps, Novo Nordisk A/S, Lundbeck A/S, ALK A/S and has had managing board memberships in Proscion A/S and Intomics A/S.
BJ has performed NIPT (fetal diagnostics) clinical diagnostic trials with Ariosa, Vanadis and Natera, with expenditures reimbursed per patient.
PWF is head of the Department of Translational Medicine at the Novo Nordisk Foundation, a non-profit philanthropic organization based in Denmark.
ÅJ, RJFL, CEW, HH, and BM have nothing to declare.
Figures

Similar articles
-
Genomic medicine and risk prediction across the disease spectrum.Crit Rev Clin Lab Sci. 2015;52(3):120-37. doi: 10.3109/10408363.2014.997930. Epub 2015 Jan 19. Crit Rev Clin Lab Sci. 2015. PMID: 25597499 Review.
-
Omics sciences and precision medicine in glioblastoma.Clin Ter. 2023 Nov-Dec;174(Suppl 2(6)):77-84. doi: 10.7417/CT.2023.2474. Clin Ter. 2023. PMID: 37994751 Review.
-
Precision medicine for suicidality: from universality to subtypes and personalization.Mol Psychiatry. 2017 Sep;22(9):1250-1273. doi: 10.1038/mp.2017.128. Epub 2017 Aug 15. Mol Psychiatry. 2017. PMID: 28809398 Free PMC article.
-
Precision Medicine and Personalized Medicine in Cardiovascular Disease.Adv Exp Med Biol. 2018;1065:589-605. doi: 10.1007/978-3-319-77932-4_36. Adv Exp Med Biol. 2018. PMID: 30051409 Review.
-
Current State of Precision Medicine in Primary Systemic Vasculitides.Front Immunol. 2019 Dec 17;10:2813. doi: 10.3389/fimmu.2019.02813. eCollection 2019. Front Immunol. 2019. PMID: 31921111 Free PMC article. Review.
Cited by
-
BMI Interacts with the Genome to Regulate Gene Expression Globally, with Emphasis in the Brain and Gut.medRxiv [Preprint]. 2024 Nov 28:2024.11.26.24317923. doi: 10.1101/2024.11.26.24317923. medRxiv. 2024. PMID: 39649609 Free PMC article. Preprint.
-
EMitool: Explainable Multi-Omics Integration for Disease Subtyping.Int J Mol Sci. 2025 Apr 30;26(9):4268. doi: 10.3390/ijms26094268. Int J Mol Sci. 2025. PMID: 40362506 Free PMC article.
-
Multi-Omics Integration of Lactylation- and PANoptosis-Based Signatures in Lung Adenocarcinoma: Prognostic Stratification and Immune Response.Int J Mol Sci. 2025 Jun 23;26(13):5999. doi: 10.3390/ijms26135999. Int J Mol Sci. 2025. PMID: 40649778 Free PMC article.
-
Omics and Multi-Omics in IBD: No Integration, No Breakthroughs.Int J Mol Sci. 2023 Oct 5;24(19):14912. doi: 10.3390/ijms241914912. Int J Mol Sci. 2023. PMID: 37834360 Free PMC article. Review.
-
Incorporating familial risk, lifestyle factors, and pharmacogenomic insights into personalized noncommunicable disease (NCD) reports for healthcare funder beneficiaries participating in the Open Genome Project.Ann Hum Genet. 2025 Jul;89(4):208-227. doi: 10.1111/ahg.12582. Epub 2024 Oct 29. Ann Hum Genet. 2025. PMID: 39470271 Free PMC article.
References
-
- Fioretos T, Wirta V, Cavelier L, et al. Implementing precision medicine in a regionally organized healthcare system in Sweden. Nat Med 2022; 28: 1980–2. - PubMed
-
- James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–858. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

