Neutralization of zoonotic retroviruses by human antibodies: Genotype-specific epitopes within the receptor-binding domain from simian foamy virus
- PMID: 37093892
- PMCID: PMC10159361
- DOI: 10.1371/journal.ppat.1011339
Neutralization of zoonotic retroviruses by human antibodies: Genotype-specific epitopes within the receptor-binding domain from simian foamy virus
Abstract
Infection with viruses of animal origin pose a significant threat to human populations. Simian foamy viruses (SFVs) are frequently transmitted to humans, in which they establish a life-long infection, with the persistence of replication-competent virus. However, zoonotic SFVs do not induce severe disease nor are they transmitted between humans. Thus, SFVs represent a model of zoonotic retroviruses that lead to a chronic infection successfully controlled by the human immune system. We previously showed that infected humans develop potent neutralizing antibodies (nAbs). Within the viral envelope (Env), the surface protein (SU) carries a variable region that defines two genotypes, overlaps with the receptor binding domain (RBD), and is the exclusive target of nAbs. However, its antigenic determinants are not understood. Here, we characterized nAbs present in plasma samples from SFV-infected individuals living in Central Africa. Neutralization assays were carried out in the presence of recombinant SU that compete with SU at the surface of viral vector particles. We defined the regions targeted by the nAbs using mutant SU proteins modified at the glycosylation sites, RBD functional subregions, and genotype-specific sequences that present properties of B-cell epitopes. We observed that nAbs target conformational epitopes. We identified three major epitopic regions: the loops at the apex of the RBD, which likely mediate interactions between Env protomers to form Env trimers, a loop located in the vicinity of the heparan binding site, and a region proximal to the highly conserved glycosylation site N8. We provide information on how nAbs specific for each of the two viral genotypes target different epitopes. Two common immune escape mechanisms, sequence variation and glycan shielding, were not observed. We propose a model according to which the neutralization mechanisms rely on the nAbs to block the Env conformational change and/or interfere with binding to susceptible cells. As the SFV RBD is structurally different from known retroviral RBDs, our data provide fundamental knowledge on the structural basis for the inhibition of viruses by nAbs. Trial registration: The study was registered at www.clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT03225794/.
Copyright: © 2023 Dynesen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


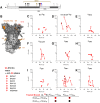


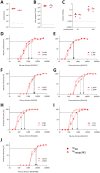
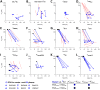
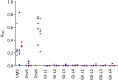


Similar articles
-
Potent neutralizing antibodies in humans infected with zoonotic simian foamy viruses target conserved epitopes located in the dimorphic domain of the surface envelope protein.PLoS Pathog. 2018 Oct 8;14(10):e1007293. doi: 10.1371/journal.ppat.1007293. eCollection 2018 Oct. PLoS Pathog. 2018. PMID: 30296302 Free PMC article.
-
An Immunodominant and Conserved B-Cell Epitope in the Envelope of Simian Foamy Virus Recognized by Humans Infected with Zoonotic Strains from Apes.J Virol. 2019 May 15;93(11):e00068-19. doi: 10.1128/JVI.00068-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894477 Free PMC article.
-
Plasma antibodies from humans infected with zoonotic simian foamy virus do not inhibit cell-to-cell transmission of the virus despite binding to the surface of infected cells.PLoS Pathog. 2022 May 23;18(5):e1010470. doi: 10.1371/journal.ppat.1010470. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35605011 Free PMC article.
-
[Mechanisms of viral emergence and interspecies transmission: the exemple of simian foamy viruses in Central Africa].Bull Acad Natl Med. 2013 Dec;197(9):1655-67; discussion 1667-8. doi: 10.1016/S0001-4079(19)31387-1. Bull Acad Natl Med. 2013. PMID: 26137812 Free PMC article. Review. French.
-
Origin, evolution and innate immune control of simian foamy viruses in humans.Curr Opin Virol. 2015 Feb;10:47-55. doi: 10.1016/j.coviro.2014.12.003. Epub 2015 Feb 17. Curr Opin Virol. 2015. PMID: 25698621 Free PMC article. Review.
Cited by
-
Structures of the Foamy virus fusion protein reveal an unexpected link with the F protein of paramyxo- and pneumoviruses.Sci Adv. 2024 Oct 11;10(41):eado7035. doi: 10.1126/sciadv.ado7035. Epub 2024 Oct 11. Sci Adv. 2024. PMID: 39392890 Free PMC article.
-
Dynamic alterations in bacterial and fungal microbiome and inflammatory cytokines following SRV-8 infection in cynomolgus monkeys.Zool Res. 2025 Mar 18;46(2):325-338. doi: 10.24272/j.issn.2095-8137.2024.278. Zool Res. 2025. PMID: 40049661 Free PMC article.
-
The crystal structure of a simian Foamy Virus receptor binding domain provides clues about entry into host cells.Nat Commun. 2023 Mar 6;14(1):1262. doi: 10.1038/s41467-023-36923-0. Nat Commun. 2023. PMID: 36878926 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

