Patient-, Clinician-, and Institution-level Variation in Inotrope Use for Cardiac Surgery: A Multicenter Observational Analysis
- PMID: 37094103
- PMCID: PMC10524016
- DOI: 10.1097/ALN.0000000000004593
Patient-, Clinician-, and Institution-level Variation in Inotrope Use for Cardiac Surgery: A Multicenter Observational Analysis
Abstract
Background: Conflicting evidence exists regarding the risks and benefits of inotropic therapies during cardiac surgery, and the extent of variation in clinical practice remains understudied. Therefore, the authors sought to quantify patient-, anesthesiologist-, and hospital-related contributions to variation in inotrope use.
Methods: In this observational study, nonemergent adult cardiac surgeries using cardiopulmonary bypass were reviewed across a multicenter cohort of academic and community hospitals from 2014 to 2019. Patients who were moribund, receiving mechanical circulatory support, or receiving preoperative or home inotropes were excluded. The primary outcome was an inotrope infusion (epinephrine, dobutamine, milrinone, dopamine) administered for greater than 60 consecutive min intraoperatively or ongoing upon transport from the operating room. Institution-, clinician-, and patient-level variance components were studied.
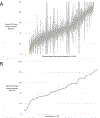
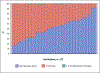
Results: Among 51,085 cases across 611 attending anesthesiologists and 29 hospitals, 27,033 (52.9%) cases received at least one intraoperative inotrope, including 21,796 (42.7%) epinephrine, 6,360 (12.4%) milrinone, 2,000 (3.9%) dobutamine, and 602 (1.2%) dopamine (non-mutually exclusive). Variation in inotrope use was 22.6% attributable to the institution, 6.8% attributable to the primary attending anesthesiologist, and 70.6% attributable to the patient. The adjusted median odds ratio for the same patient receiving inotropes was 1.73 between 2 randomly selected clinicians and 3.55 between 2 randomly selected institutions. Factors most strongly associated with increased likelihood of inotrope use were institutional medical school affiliation (adjusted odds ratio, 6.2; 95% CI, 1.39 to 27.8), heart failure (adjusted odds ratio, 2.60; 95% CI, 2.46 to 2.76), pulmonary circulation disorder (adjusted odds ratio, 1.72; 95% CI, 1.58 to 1.87), loop diuretic home medication (adjusted odds ratio, 1.55; 95% CI, 1.42 to 1.69), Black race (adjusted odds ratio, 1.49; 95% CI, 1.32 to 1.68), and digoxin home medication (adjusted odds ratio, 1.48; 95% CI, 1.18 to 1.86).
Conclusions: Variation in inotrope use during cardiac surgery is attributable to the institution and to the clinician, in addition to the patient. Variation across institutions and clinicians suggests a need for future quantitative and qualitative research to understand variation in inotrope use affecting outcomes and develop evidence-based, patient-centered inotrope therapies.
Copyright © 2023 American Society of Anesthesiologists. All Rights Reserved.
Conflict of interest statement
The authors declare: Dr. Mathis has received a research grant from the US National Institutes of Health (NHLBI K01HL141701). Dr. Janda has received a research grant from the US National Institutes of Health (NIGMS T32GM103730), has received research support paid to the University of Michigan, and unrelated to this present work, from Becton, Dickinson and Company. Dr. Schonberger has received a research grant from the US National institutes of Health (NIA R01AG059607). Dr. Schonberger reports owning stock in Johnson & Johnson unrelated to the present work. Dr. Schonberger reports that his organization receives funding from Merck, Inc. for a study in which he is co-investigator unrelated to the present work. Dr. Shook reports support from Edwards Lifesciences and UpToDate unrelated to the present work and has provided expert review and testimony unrelated to the present work. Dr. Muehlschlegel has received research grants from the US National Institutes of Health (R01HL14998, R01HL150401) unrelated to the present work. No other relationships or activities that could appear to have influenced the submitted work are reported.
Figures


References
-
- D’Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, Shahian DM: The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018; 105:15–23 - PubMed
-
- Camaj A, Zahuranec DB, Paone G, Benedetti BR, Behr WD, Zimmerman MA, Zhang M, Kramer RS, Penn J, Theurer PF, Paugh TA, Engoren M, DeLucia A 3rd, Prager RL, Likosky DS, Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative: Organizational Contributors to the Variation in Red Blood Cell Transfusion Practices in Cardiac Surgery: Survey Results From the State of Michigan. Anesth Analg 2017; 125:975–80 - PMC - PubMed
-
- Landoni G, Greco T, Biondi-Zoccai G, Nigro Neto C, Febres D, Pintaudi M, Pasin L, Cabrini L, Finco G, Zangrillo A: Anaesthetic drugs and survival: a Bayesian network meta-analysis of randomized trials in cardiac surgery. Br J Anaesth 2013; 111:886–96 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

