Therapeutic Potential of Human Intestinal Organoids in Tissue Repair Approaches in Inflammatory Bowel Diseases
- PMID: 37094358
- PMCID: PMC10472753
- DOI: 10.1093/ibd/izad044
Therapeutic Potential of Human Intestinal Organoids in Tissue Repair Approaches in Inflammatory Bowel Diseases
Abstract
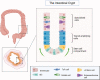
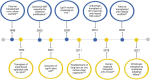
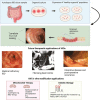
Inflammatory bowel diseases (IBDs) are chronic immune-mediated conditions characterized by significant gut tissue damage due to uncontrolled inflammation. Anti-inflammatory treatments have improved, but there are no current prorepair approaches. Organoids have developed into a powerful experimental platform to study mechanisms of human diseases. Here, we specifically focus on its role as a direct tissue repair modality in IBD. We discuss the scientific rationale for this, recent parallel advances in scientific technologies (CRISPR [clustered regularly interspaced short palindromic repeats]/Cas9 and metabolic programming), and in addition, the clinical IBD context in which this therapeutic approach is tractable. Finally, we review the translational roadmap for the application of organoids and the need for this as a novel direction in IBD.
Keywords: CD; IBD; UC; organoids; repair; stem cells.
Plain language summary
We provide an overview of the translational potential of human intestinal organoids as a prorepair therapy in inflammatory bowel disease. We focus on the key areas of clinical application and the necessary steps toward tangible progress in this novel approach.
© 2023 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
Both authors have no disclosures or conflicts of interest to declare.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

