Gold-Enhanced Brachytherapy by a Nanoparticle-Releasing Hydrogel and 3D-Printed Subcutaneous Radioactive Implant Approach
- PMID: 37094373
- PMCID: PMC11469283
- DOI: 10.1002/adhm.202300305
Gold-Enhanced Brachytherapy by a Nanoparticle-Releasing Hydrogel and 3D-Printed Subcutaneous Radioactive Implant Approach
Abstract
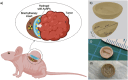
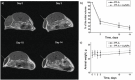
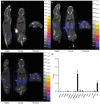
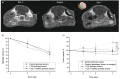
Brachytherapy (BT) is a widely used clinical procedure for localized cervical cancer treatment. In addition, gold nanoparticles (AuNPs) have been demonstrated as powerful radiosensitizers in BT procedures. Prior to irradiation by a BT device, their delivery to tumors can enhance the radiation effect by generating low-energy photons and electrons, leading to reactive oxygen species (ROS) production, lethal to cells. No efficient delivery system has been proposed until now for AuNP topical delivery to localized cervical cancer in the context of BT. This article reports an original approach developed to accelerate the preclinical studies of AuNP-enhanced BT procedures. First, an AuNP-containing hydrogel (Pluronic F127, alginate) is developed and tested in mice for degradation, AuNP release, and biocompatibility. Then, custom-made 3D-printed radioactive BT inserts covered with a AuNP-containing hydrogel cushion are designed and administered by surgery in mice (HeLa xenografts), which allows for measuring AuNP penetration in tumors (≈100 µm), co-registered with the presence of ROS produced through the interactions of radiation and AuNPs. Biocompatible AuNPs-releasing hydrogels could be used in the treatment of cervical cancer prior to BT, with impact on the total amount of radiation needed per BT treatment, which will result in benefits to the preservation of healthy tissues surrounding cancer.
Keywords: 3D printing; brachytherapy; gold nanoparticles; hydrogels; localized vaginal delivery; radiosensitizers; reactive oxygen species.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Abraham J., Gulley J. L., The Bethesda Handbook of Clinical Oncology, Lippincott Williams & Wilkins, Philadelphia, United States: 2022.
-
- de la Puente P., Azab A. K., J. Controlled Release 2014, 192, 19. - PubMed

