Identification and structure-activity relationships for a series of N, N-disubstituted 2-aminobenzothiazoles as potent inhibitors of S. aureus
- PMID: 37094726
- PMCID: PMC10257494
- DOI: 10.1016/j.bmcl.2023.129301
Identification and structure-activity relationships for a series of N, N-disubstituted 2-aminobenzothiazoles as potent inhibitors of S. aureus
Abstract
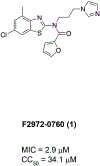
An internal collection of commercial and synthetically derived small molecule compounds was screened against several drug-resistant bacterial pathogens. Compound 1, a known N, N-disubstituted 2-aminobenzothiazole, was found to be a potent inhibitor of Staphylococcus aureus and several associated clinically relevant strains of methicillin-resistant S. aureus suggesting a possible novel mechanism of inhibition. It failed to show activity in any of the Gram-negative pathogens it was tested in. Evaluation in Escherichia coli BW25113 and Pseudomonas aeruginosa PAO1, as well as in their respective hyperporinated and efflux pump-deletion mutants revealed that activity in Gram-negative bacteria is diminished because this benzothiazole scaffold is a substrate for bacterial efflux pumps. Several analogs of 1 were synthesized to generate basic structure-activity relationships for the scaffold which highlighted that the N-propyl imidazole moiety was critical for the observed antibacterial activity.
Keywords: 2-Aminobenzothiazole; Antibacterial; Methicillin-resistant S. aureus; Multidrug efflux pump; Staphylococcus aureus.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Some authors are inventors on a composition of matter patent that cover several of the compounds described here.
Figures







References
-
- Organization(WHO), W. H. Antimicrobial Resistance https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
-
- CDC, Antibiotic Resistance Threats in the United States, 2019. U.S. Department of Health and Human Services, CDC: Atlanta, GA, 2019.
-
- Martens E; Demain AL, The antibiotic resistance crisis, with a focus on the United States. The Journal of Antibiotics 2017, 70 (5), 520–526. - PubMed
-
- Church NA; McKillip JL, Antibiotic resistance crisis: challenges and imperatives. Biologia 2021, 76 (5), 1535–1550.
-
- Serra-Burriel M; Keys M; Campillo-Artero C; Agodi A; Barchitta M; Gikas A; Palos C; Lopez-Casasnovas G, Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS One 2020, 15 (1), e0227139. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

