Fecal transplantation for treatment of inflammatory bowel disease
- PMID: 37094824
- PMCID: PMC10133790
- DOI: 10.1002/14651858.CD012774.pub3
Fecal transplantation for treatment of inflammatory bowel disease
Abstract
Background: Inflammatory bowel disease (IBD) is a chronic, relapsing disease of the gastrointestinal (GI) tract that is thought to be associated with a complex interplay between the immune system, the GI tract lining, the environment, and the gut microbiome, leading to an abnormal inflammatory response in genetically susceptible individuals. An altered composition of the gut's native microbiota, known as dysbiosis, may have a major role in the pathogenesis of ulcerative colitis (UC) and Crohn disease (CD), two subtypes of IBD. There is growing interest in the correction of this underlying dysbiosis using fecal microbiota transplantation (FMT).
Objectives: To evaluate the benefits and safety profile of FMT for treatment of IBD in adults and children versus autologous FMT, placebo, standard medication, or no intervention.
Search methods: We searched CENTRAL, MEDLINE, Embase, two clinical trial registries, and the reference sections of published trials through 22 December 2022.
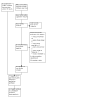
Selection criteria: We included randomized controlled trials that studied adults and children with UC or CD. Eligible intervention arms used FMT, defined as the delivery of healthy donor stool containing gut microbiota to a recipient's GI tract, to treat UC or CD.
Data collection and analysis: Two review authors independently screened studies for inclusion. Our primary outcomes were: 1. induction of clinical remission, 2. maintenance of clinical remission, and 3. serious adverse events. Our secondary outcomes were: 4. any adverse events, 5. endoscopic remission, 6. quality of life, 7. clinical response, 8. endoscopic response, 9. withdrawals, 10. inflammatory markers, and 11. microbiome outcomes. We used the GRADE approach to assess the certainty of evidence.
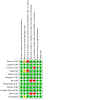
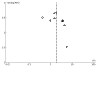
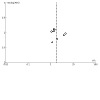
Main results: We included 12 studies with 550 participants. Three studies were conducted in Australia; two in Canada; and one in each of the following: China, the Czech Republic, France, India, the Netherlands, and the USA. One study was conducted in both Israel and Italy. FMT was administered in the form of capsules or suspensions and delivered by mouth, nasoduodenal tube, enema, or colonoscopy. One study delivered FMT by both oral capsules and colonoscopy. Six studies were at overall low risk of bias, while the others had either unclear or high risk of bias. Ten studies with 468 participants, of which nine studies focused on adults and one focused on children, reported induction of clinical remission in people with UC at longest follow-up (range 6 to 12 weeks) and showed that FMT may increase rates of induction of clinical remission in UC compared to control (risk ratio (RR) 1.79, 95% confidence interval (CI) 1.13 to 2.84; low-certainty evidence). Five studies showed that FMT may increase rates of induction of endoscopic remission in UC at longest follow-up (range 8 to 12 weeks); however, the CIs around the summary estimate were wide and included a possible null effect (RR 1.45, 95% CI 0.64 to 3.29; low-certainty evidence). Nine studies with 417 participants showed that FMT may result in little to no difference in rates of any adverse events (RR 0.99, 95% CI 0.85 to 1.16; low-certainty evidence). The evidence was very uncertain about the risk of serious adverse events (RR 1.77, 95% CI 0.88 to 3.55; very low-certainty evidence) and improvement in quality of life (mean difference (MD) 15.34, 95% CI -3.84 to 34.52; very low-certainty evidence) when FMT was used to induce remission in UC. Two studies, of which one also contributed data for induction of remission in active UC, assessed maintenance of remission in people with controlled UC at longest follow-up (range 48 to 56 weeks). The evidence was very uncertain about the use of FMT for maintenance of clinical remission (RR 2.97, 95% CI 0.26 to 34.42; very low-certainty evidence) and endoscopic remission (RR 3.28, 95% CI 0.73 to 14.74; very low-certainty evidence). The evidence was also very uncertain about the risk of serious adverse events, risk of any adverse events, and improvement in quality of life when FMT was used to maintain remission in UC. None of the included studies assessed use of FMT for induction of remission in people with CD. One study with 21 participants reported data on FMT for maintenance of remission in people with CD. The evidence was very uncertain about the use of FMT for maintenance of clinical remission in CD at 24 weeks (RR 1.21, 95% CI 0.36 to 4.14; very low-certainty evidence). The evidence was also very uncertain about the risk of serious or any adverse events when FMT was used to maintain remission in CD. None of the studies reported data on use of FMT for maintenance of endoscopic remission or improvement in quality of life in people with CD.
Authors' conclusions: FMT may increase the proportion of people with active UC who achieve clinical and endoscopic remission. The evidence was very uncertain about whether use of FMT in people with active UC impacted the risk of serious adverse events or improvement in quality of life. The evidence was also very uncertain about the use of FMT for maintenance of remission in people with UC, as well as induction and maintenance of remission in people with CD, and no conclusive statements could be made in this regard. Further studies are needed to address the beneficial effects and safety profile of FMT in adults and children with active UC and CD, as well as its potential to promote longer-term maintenance of remission in UC and CD.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AI: none.
NP: none.
MZ: none.
NZM: none.
ETS: none.
OG: none.
SA: none.
MN: none.
Figures








































Update of
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2018 Nov 13;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD012774. doi: 10.1002/14651858.CD012774.pub3. PMID: 30480772 Free PMC article. Updated.
References
References to studies included in this review
Březina 2021 {published data only (unpublished sought but not used)}
Costello 2019 {published data only}
Crothers 2021 {published data only}
-
- NCT02390726. Fecal Microbiota Transplant in the Treatment of Ulcerative Colitis (FMTUC). clinicaltrials.gov/ct2/show/NCT02390726 (first received 17 March 2015).
Fang 2021 {published data only}
Haifer 2022 {published data only}
-
- ACTRN12619000611123. Lyophilised oral faecal microbiota transplantation in the management of ulcerative colitis (LOTUS Study). www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377325 (first received 3 April 2019).
-
- Haifer C, Paramsothy S, Kaakoush NO, Saikal A, Ghaly S, Yang T, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterology and Hepatology 2022;7(2):141-51. [PMID: ] - PubMed
Moayyedi 2015 {published and unpublished data}
-
- Moayyedi P, Surette M, Wolfe M, Taraschi R, Kim P, Libertucci J, et al. A randomized, placebo controlled trial of fecal microbiota therapy in active ulcerative colitis. Gastroenterology 2014;146(5):S-159.
-
- Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149(1):102-9. [PMID: ] - PubMed
Pai 2021 {published data only}
-
- NCT02487238. Pediatric FEcal Microbiota Transplant for Ulcerative Colitis (PediFETCh). clinicaltrials.gov/ct2/show/NCT02487238 (first received 1 July 2015).
-
- Pai N, Popov J, Hill L, Hartung E, Grzywacz K, Moayyedi P, et al. Results of the first pilot randomized controlled trial of fecal microbiota transplant in pediatric ulcerative colitis: lessons, limitations, and future prospects. Gastroenterology 2021;161(2):388-93. [PMID: ] - PubMed
-
- Pai N, Popov J, Lee C. A randomized, placebo-controlled trial of fecal microbial transplantation for pediatric ulcerative colitis [Pedifetch Trial]. Journal of Pediatric Gastroenterology and Nutrition 2016;63:S79-80.
-
- Popov J, Hartung E, Hill L, Chauhan U, Pai N. Fecal microbiota transplantation: perceptions and experiences in a pediatric ulcerative colitis population (PediFETCH trial). Gastroenterology 2019;156(6):S-458.
Paramsothy 2017 {published data only}
-
- Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389(10075):1218-28. [PMID: ] - PubMed
-
- Paramsothy S, Kamm MA, Walsh A, den Bogaerde J, Samuel D, Leong RW, et al. Multi donor intense faecal microbiota transplantation is an effective treatment for resistant ulcerative colitis: a randomised placebo-controlled trial. Gastroenterology 2016;150(4):S122-3.
-
- Paramsothy S, Kamm MA, Walsh AJ, den Bogaerde J, Samuel D, Leong RW, et al. Multi donor intense faecal microbiota transplantation is an effective treatment for resistant ulcerative colitis: a randomised placebo controlled trial and microbiota analysis. Journal of Gastroenterology and Hepatology (Australia) 2016;31:143.
Rossen 2015 {published data only}
-
- Rossen NG, Fuentes S, Spek MJ, Tijssen JG, Hartman JH, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149(1):110-8. [PMID: ] - PubMed
Sarbagili Shabat 2022 {unpublished data only}
-
- NCT02734589. Fecal transplantation using a diet for donor and recipient in refractory colitis. clinicaltrials.gov/ct2/show/NCT02734589 (first received 12 April 2016).
Sokol 2020 {published data only}
Sood 2019a {published data only (unpublished sought but not used)}
-
- Midha V, Singh A, Mahajan R, Mehta V, Khattar H, Narang V, et al. Maintenance with faecal microbiota transplantation enhances deep remission in patients with ulcerative colitis in clinical remission. Gut 2018;67:A74.
-
- Sood A, Mahajan R, Singh A, Midha V, Mehta V, Narang V, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. Journal of Crohn's and Colitis 2019;13(10):1311-7. [PMID: ] - PubMed
References to studies excluded from this review
Adler 2019 {published data only}
-
- Adler E, Tabaa A, Kassam Z, Zydek M, Terdiman J, El-Nachef N. Capsule-delivered fecal microbiota transplant is safe and well tolerated in patients with ulcerative colitis. Digestive Diseases and Sciences 2019;64(9):2452-4. - PubMed
Allegretti 2016 {published data only}
-
- Allegretti JR, Kassam Z, Smith M, Korzenik JR, Chan WW. Irregular bowel movements following fecal microbiota transplantation (FMT) are associated with pre-existing irritable bowel syndrome but not FMT-related factors. Gastroenterology 2016;150(4):S742.
Borody 2003 {published data only}
-
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. Journal of Clinical Gastroenterology 2003;37(1):42-7. - PubMed
Chen 2018 {published data only}
-
- Chen Q, Fan Y, Zhang B, Huang Q, Xiao C, Xu H, et al. Faecal microbiota transplantation by capsules for active uncreative colitis: a randomised trial. Gut 2018;67:A76-7.
Chen 2020 {published data only}
Chin 2017 {published data only}
Ding 2019 {published data only}
-
- Ding X, Li Q, Li P, Zhang T, Cui B, Ji G, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Safety 2019;42(7):869-80. - PubMed
El‐Nachef 2020 {published data only}
-
- El‐Nachef N, Smith B, Piceno Y, Zydek M, Syriani L, Terdiman JP, et al. Antibiotic pretreatment prior to fecal microbiota transplantation increases rates of remission in patients with mild to moderate ulcerative colitis: results from a pilot randomized clinical trial. Gastroenterology 2020;158(6):S-14.
Fang 2017 {published data only}
-
- Fang YH, Chen J, Yu JD, Luo YY, Lou JG. The preliminary investigation of faecal microbiota transplantation for paediatric recurrent chronic bowel diseases and literature review. Hong Kong Journal of Paediatrics 2017;22:199-203.
Fischer 2016 {published data only}
-
- Fischer M, Kao D, Kelly C, Kuchipudi A, Jafri SM, Blumenkehl M, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory clostridium difficile infection in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2016;22(10):2402-9. - PubMed
Gionchetti 2000 {published data only}
-
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119(2):305-9. - PubMed
Hourigan 2015 {published data only}
-
- Hourigan SK, Chen LA, Grigoryan Z, Laroche G, Weidner M, Sears CL, et al. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2015;42(6):741-52. - PubMed
Ishikawa 2017a {published data only}
-
- Ishikawa D, Sasaki T, Osada T, Kuwahara-Arai K, Haga K, Shibuya T, et al. Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflammatory Bowel Diseases 2017;23(1):116-25. - PubMed
Ishikawa 2017b {published data only}
-
- Ishikawa D, Takahashi M, Haga K, Shibuya T, Osada T, Watanabe S. Combination therapy of fresh fecal microbial transplantation and antibiotics for ulcerative colitis. Journal of Crohn's and Colitis 2017;11(Suppl 1):S472. - PubMed
Ishikawa 2019 {published data only (unpublished sought but not used)}
-
- Ishikawa D, Sasaki T, Takahashi M, Okahara K, Ito S, Haga K, et al. Combination therapy of fresh fecal microbial transplantation and triple-antibiotic therapy for ulcerative colitis. American Journal of Gastroenterology 2019;114:S14.
Ishikawa 2022 {published data only}
-
- Ishikawa D, Zhang X, Nomura K, Seki N, Haraikawa M, Haga K, et al. A randomized placebo-controlled trial of combination therapy with post-triple-antibiotic-therapy fecal microbiota transplantation and alginate for ulcerative colitis: protocol. Frontiers in Medicine (Lausanne) 2022;9:779205. - PMC - PubMed
Karolewska‐Bochenek 2018 {published data only}
-
- Karolewska-Bochenek K, Grzesiowski P, Banaszkiewicz A, Gawronska A, Kotowska M, Dziekiewicz M, et al. A two-week fecal microbiota transplantation course in pediatric patients with inflammatory bowel disease. Advances in Experimental Medicine and Biology 2018;1047:81-7. - PubMed
Kedia 2022 {published data only}
-
- Kedia S, Virmani S, Vuyyuru SK, Kumar P, Kante B, Sahu P, et al. Combination of fecal microbiota transplantation and anti-inflammatory diet induces clinical and deep remission in patients with mild-moderate ulcerative colitis. Gastroenterology 2022;162(7):S-1391.
-
- Kedia S, Virmani S, Vuyyuru SK, Kumar P, Kante B, Sahu P, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut 2022;71(12):2401-13. - PubMed
Kump 2018 {published data only}
-
- Kump P, Wurm P, Gröchenig HP, Wenzl H, Petritsch W, Halwachs B, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Alimentary Pharmacology and Therapeutics 2018;47(1):67-77. - PMC - PubMed
Landy 2013 {published data only}
-
- Landy J, Al-Hassi HO, Mann ER, Peake ST, McLaughlin SD, Perry-Woodford ZL, et al. A prospective controlled pilot study of fecal microbiota transplantation for chronic refractory pouchitis. Gut 2013;62:A162.
Li 2020 {published data only}
-
- Li Q, Zhang T, Ding X, Xiang L, Cui B, Buch H, et al. Enhancing patient adherence to fecal microbiota transplantation maintains the long-term clinical effects in ulcerative colitis. European Journal of Gastroenterology and Hepatology 2020;32(8):955-62. - PubMed
-
- NCT01790061. Standardized fecal microbiota transplantation for ulcerative colitis. www.clinicaltrials.gov/ct2/show/NCT01790061 (first received 13 February 2013).
Ma 2020 {published data only}
-
- Ma J, Hudak J, Akbari P, Papkoff J. A rationally selected, orally administered, live biotherapeutic confortium of commensial bacteria for the treatment of ulcerative colitis. Gastroenterology 2020;158(6):S-472-3.
Mahajan 2018 {published data only}
-
- Mahajan R, Midha V, Mehta V, Singh A, Khattar H, Gupta Y, et al. Efficacy of faecal microbiota therapy in patients with steroid dependent active ulcerative colitis. Gut 2018;67:A74.
Mandalia 2016 {published data only}
Michail 2018 {published data only}
-
- Michail S. Fecal microbial transplant in children with ulcerative colitis: a randomized, double-blinded, placebo-controlled pilot study: retracted. Journal of Pediatric Gastroenterology and Nutrition 2018;10.1097/MPG.0000000000001772:Study retracted. [DOI: 10.1097/MPG.0000000000001772] - DOI - PubMed
-
- NCT02291523. The effect of therapeutic fecal transplant on the gut microbiome in children with ulcerative colitis (FMT_UC). www.clinicaltrials.gov/ct2/show/NCT02291523 (first received 14 November 2014).
Mintz 2016 {published data only}
-
- Mintz M, Monzur F, Chowdhury T, Rowehl L, Grewal S, Li E, et al. Comparing fecal microbial transplant outcomes in patients with recurrent Clostridium difficile or ulcerative colitis. Inflammatory Bowel Diseases 2016;22(Suppl 1):S31.
NCT04436874 {published data only}
-
- NCT04436874. Fecal microbiota transplantation in the treatment of inflammatory bowel disease (FMT-IBD). clinicaltrials.gov/ct2/show/NCT04436874 (first received 18 June 2020).
NCT05202990 {published data only}
-
- NCT05202990. Oral fecal microbiota transplantation in pediatric ulcerative colitis (T-FORE). clinicaltrials.gov/ct2/show/NCT05202990 (first received 24 January 2022).
Okahara 2020 {published data only}
Osaki 2021 {published data only}
-
- Osaki H, Jodai Y, Koyama K, Omori T, Horiguchi N, Kamano T, et al. Clinical response and changes in the fecal microbiota and metabolite levels after fecal microbiota transplantation in patients with inflammatory bowel disease and recurrent Clostridioides difficile infection. Fujita Medical Journal 2021;7(3):87-98. - PMC - PubMed
Quraishi 2019 {published data only}
-
- Quraishi MN, Yalchin M, Blackwell C, Segal J, Sharma N, Hawkey P, et al. STOP-Colitis pilot trial protocol: a prospective, open-label, randomised pilot study to assess two possible routes of faecal microbiota transplant delivery in patients with ulcerative colitis. BMJ Open 2019;9(11):e030659. - PMC - PubMed
Quraishi 2022 {published data only}
-
- Quraishi N, Quince C, Hewitt C, Beggs A, Gerasimidis K, Sharma N, et al. Novel interactions between bacterial strains and mucosal immune profiles after FMT for UC: STOP-colitis results. Gut 2022;71:A28.
Rainer 2018 {published data only}
-
- Rainer F, Blesl A, Petritsch W, Wenzl HH, Baumann-Durchschein F, Posch A, et al. Frozen donor stool for fecal microbiota transplantation is as effective as fresh stool in inducing response and remission in active ulcerative colitis. United European Gastroenterology Journal 2018;6(Suppl 8):A69-70.
Silber 2022 {published data only}
-
- Silber J, Norman J, Kanno T, Crossette E, Szabady R, Menon R, et al. Randomized, double-blind, placebo (pbo)-controlled, single- and multiple-dose phase 1 study of VE202, a defined bacterial consortium for treatment of IBD: safety and colonization dynamics of a novel live biotherapeutic product (LBP) in healthy adults. Gastroenterology 2022;162(3):S65-6.
Smith 2022 {published data only}
-
- NCT03006809. Optimal fecal microbiota transplant dosing for mild to moderate ulcerative colitis. clinicaltrials.gov/ct2/show/NCT03006809 (first received 30 December 2016).
Sood 2019b {published data only}
Steube 2019 {published data only}
-
- Steube A, Vital M, Grunert P, Pieper DH, Stallmach A. Long-term multidonor faecal microbiota transfer by oral capsules for active ulcerative colitis. Journal of Crohn's and Colitis 2019;13(11):1480-1. - PubMed
Tian 2019 {unpublished data only}
-
- NCT03016780. Fecal microbiota transplantation for ulcerative colitis (FMTFUC). clinicaltrials.gov/ct2/show/NCT03016780 (first received 11 January 2017).
UMIN000025846 {published data only}
-
- UMIN000025846. A trial of a combination therapy of fecal microbial transplantation and antibiotics for inflammatory bowel disease. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_his_list.cgi?recptno=R000029717 (first received 1 February 2017).
UMIN000026485 {published data only}
-
- UMIN000026485. Research on the efficacy of fecal microbiota transplantation and microbiota in Japanese children with ulcerative colitis. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000030424 (first received 9 March 2017).
UMIN000041968 {published data only}
-
- UMIN000041968. Single center randomized study: alginate combined fecal microbiota transplantation for ulcerative colitis. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000047906 (first received 1 October 2020).
Wei 2016 {published data only}
Xiang 2020 {published data only}
-
- NCT01793831. Standardized fecal microbiota transplantation for Crohn's disease [Efficacy and safety of standardized fecal microbiota transplantation for moderate to severe Crohn's disease]. clinicaltrials.gov/ct2/show/NCT01793831 (first received 18 February 2013).
Yang 2020 {published data only}
-
- Yang Z, Bu C, Yuan W, Shen Z, Quan Y, Wu S, et al. Fecal microbiota transplant via endoscopic delivering through small intestine and colon: no difference for Crohn's disease. Digestive Diseases and Sciences 2020;65(1):150-7. - PubMed
-
- Yang Z, Wang X, Bu C. Fecal microbiota transplant for Crohn's disease: a prospective, randomized study in Chinese population. United European Gastroenterology Journal 2017;5(Suppl 5):A112-3.
References to studies awaiting assessment
Caenepeel 2022 {published data only}
-
- Caenepeel C, Deleu S, Arnauts K, Vázquez-Castellanos JF, Braekeleire S, Machiels K, et al. Standardized faecal microbiota transplantation with microbiome-guided donor selection in active UC patients: a randomized, placebo-controlled intervention study. Journal of Crohn's and Colitis 2022;16(Suppl 1):i003-4.
-
- Deleu S, Caenepeel C, Arnauts K, Vázquez-Castellanos JF, Braekeleire S, Machiels K, et al. Standardized fecal microbiota transplantation through microbiome-guided donor selection in active ulcerative colitis patients: a randomized, placebo-controlled intervention study. Gastroenterology 2022;162(7):S-95-6.
Jitsumura 2022 {published data only}
-
- Jitsumura M, Cunningham A, Hitchings MD, Wilkinson T, Kinross J, Row P, et al. Faecal microbiota transplant in ulcerative colitis (FMTUC) – a randomised clinical trial feasibility study. Journal of Crohn's and Colitis 2022;16(Suppl 1):i612-3.
NCT02272868 {published data only}
-
- NCT02272868. Fecal microbial transplant in pediatric Crohn's disease (FMTCD). clinicaltrials.gov/ct2/show/NCT02272868 (first received 23 October 2014).
Zhang 2019 {published data only}
-
- Zhang KQ, Jiang Q, Zhang HB. Effect of fecal microbiota transplantation on gastrointestinal function and intestinal flora in patients with ulcerative colitis. Shijie Huaren Xiaohua Zazhi 2019;27(1):68-72.
References to ongoing studies
CTRI/2021/03/032131 {published data only}
-
- CTRI/2021/03/032131. Stool transplant for treatment of colitis and Crohns disease. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/03/032131 (first received 18 March 2021).
EUCTR 2019‐003816‐29 {published data only}
-
- EUCTR 2019-003816-29. Fecal microbiota transplantation in Crohn's disease as relay after anti-TNF withdrawal. www.clinicaltrialsregister.eu/ctr-search/trial/2019-003816-29/FR (first received 14 October 2019).
NCT01961492 {published data only}
-
- NCT01961492. Fecal microbiota transplantation in patients with ulcerative colitis. clinicaltrials.gov/ct2/show/NCT01961492 (first received 11 October 2013).
NCT02335281 {published data only}
-
- NCT02335281. Standardized fecal microbiota transplantation for inflammatory bowel disease (SFMT-IBD). clinicaltrials.gov/ct2/show/NCT02335281 (first received 9 January 2015).
NCT02998112 {published data only}
-
- NCT02998112. Fecal microbiota transplantation for ulcerative colitis through colonic transendoscopic enteral tubing. clinicaltrials.gov/ct2/show/NCT02998112 (first received 20 December 2016).
NCT03078803 {published data only}
-
- NCT03078803. Fecal transplant for Crohn's disease. clinicaltrials.gov/ct2/show/NCT03078803 (first received 13 March 2017).
NCT03110289 {published data only}
-
- NCT03110289. Restoration of the microbiome through superdonor selection (RESTORE-UC). clinicaltrials.gov/ct2/show/NCT03110289 (first received 12 April 2017).
NCT03273465 {published data only}
-
- NCT03273465. Fecal microbiota transplantation in ulcerative colitis. clinicaltrials.gov/ct2/show/NCT03273465 (first received 6 September 2017).
NCT03483246 {published data only}
-
- NCT03483246. Impact of fecal microbiota transplantation in ulcerative colitis (REBALANCE-UC). clinicaltrials.gov/ct2/show/NCT03483246 (first received 30 March 2018).
NCT03561532 {published data only}
-
- NCT03561532. Fecal transplantation in ulcerative colitis (FMT-CU). clinicaltrials.gov/ct2/show/NCT03561532 (first received 19 June 2018).
NCT03582969 {published data only}
-
- NCT03582969. Capsulized fecal microbiota transplantation in pediatric ulcerative colitis patients (FMT UC). clinicaltrials.gov/ct2/show/NCT03582969 (first received 11 July 2018).
NCT03716388 {published data only}
-
- NCT03716388. Fecal microbiota therapy vs 5-aminosalicylates for induction of remission in newly diagnosed mild-moderately active UC. clinicaltrials.gov/ct2/show/NCT03716388 (first received 23 October 2018).
NCT03804931 {published data only}
-
- NCT03804931. Fecal microbiota transplantation for ulcerative colitis. clinicaltrials.gov/ct2/show/NCT03804931 (first received 15 January 2019).
NCT03998488 {published data only}
-
- NCT03998488. Examining the efficacy of fecal microbiota transplantation (FMT) and dietary fiber in patients with ulcerative colitis (MINDFUL). clinicaltrials.gov/ct2/show/NCT03998488 (first received 26 June 2019).
NCT04034758 {published data only}
-
- NCT04034758. Safety and efficacy of heterologous FMT by SQIMC-md capsule in mild-moderate ulcerative colitis patients (SQIMC-md). clinicaltrials.gov/ct2/show/NCT04034758 (first received 26 July 2019).
NCT04202211 {published data only}
-
- NCT04202211 [FMT for remission of active ulcerative colitis in adults]. clinicaltrials.gov/ct2/show/NCT04202211 (first received 17 December 2019).
NCT04328922 {published data only}
-
- NCT04328922. Fecal microbial transplantation and vedolizumab treatment of Crohn's disease. clinicaltrials.gov/ct2/show/NCT04328922 (first received 1 April 2020).
NCT04373473 {published data only}
-
- NCT04373473. Evaluation of the safety and efficacy of lyophilized fecal microbiota transplantation administered orally for prevention of relapse or intestinal inflammation in adults with ulcerative colitis. clinicaltrials.gov/ct2/show/NCT04373473 (first received 4 May 2020).
NCT04434872 {published data only}
-
- NCT04434872. Fecal microbiota transplantation as a treatment for ulcerative colitis. clinicaltrials.gov/ct2/show/NCT04434872 (first received 17 June 2020).
NCT04521205 {published data only}
-
- NCT03426683. The effect of intestinal microbiota transplantation for inflammatory bowel diseases (IBD). clinicaltrials.gov/ct2/show/NCT03426683 (first received 8 February 2018).
-
- NCT04521205. A multicenter clinical trial: efficacy, safety of fecal microbiota transplantation for inflammatory bowel disease. clinicaltrials.gov/ct2/show/NCT04521205 (first received 20 August 2020).
NCT04637438 {published data only}
-
- NCT04637438. Fecal microbiota transplantation in postoperative Crohn's disease. clinicaltrials.gov/ct2/show/NCT04637438 (first received 19 November 2020).
NCT04924270 {published data only}
-
- NCT04924270. Safety and efficacy of faecal microbiota transplantation in treatment-naïve patients with newly diagnosed chronic inflammatory diseases (FRONT). clinicaltrials.gov/ct2/show/NCT04924270 (first received 11 June 2021).
NCT04970446 {published data only}
-
- NCT04970446. The MIRO II study: microbial restoration in inflammatory bowel diseases (MIRO II). clinicaltrials.gov/ct2/show/NCT04970446 (first received 21 July 2021).
NCT04997733 {published data only}
-
- NCT04997733. Fecal microbiota transplantation in Crohn's disease as relay after anti-TNF withdrawal (MIRACLE). clinicaltrials.gov/ct2/show/NCT04997733 (first received 10 August 2021).
NCT05030064 {published data only}
-
- NCT05030064. Clinical study on the fecal microbiota transplantation in the treatment of ulcerative colitis with depression. clinicaltrials.gov/ct2/show/NCT05030064 (first received 1 September 2021).
NCT05538026 {published data only}
-
- NCT05538026. Effectiveness of fecal microbiota transplantation as add-on therapy in mild-to-moderate ulcerative colitis. clinicaltrials.gov/ct2/show/NCT05538026 (first received 13 September 2022).
Pai 2019 {published data only}
Stallmach 2022 {published data only}
-
- Stallmach A, Grunert P, Stallhofer J, Löffler B, Baier M, Rödel J, et al. Transfer of FRozen Encapsulated multi-donor Stool filtrate for active ulcerative Colitis (FRESCO): study protocol for a prospective, multicenter, double-blind, randomized, controlled trial. Trials 2022;23(1):173. - PMC - PubMed
UMIN000033127 {published data only}
-
- UMIN000033127. Multicenter randomized controlled trial to study the efficacy of multidonor fecal microbiota transplantation for Crohn's disease. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000037777 (first received 24 June 2018).
Additional references
Abraham 2009
Abraham 2012
Ahuja 2010
-
- Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. Journal of Digestive Diseases 2010;11(3):134-47. - PubMed
Alang 2015
Ananthakrishnan 2015
-
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nature Reviews Gastroenterology and Hepatology 2015;12(4):205-17. - PubMed
Anderson 2012
-
- Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2012;36(6):503-16. - PubMed
Armuzzi 2012
-
- Armuzzi A, Assche G, Reinisch W, Pineton de Chambrun G, Griffiths A, Sladek M, et al. Results of the 2nd scientific workshop of the ECCO (IV): therapeutic strategies to enhance intestinal healing in inflammatory bowel disease. Journal of Crohn's and Colitis 2012;6(4):492-502. - PubMed
Assa 2016
-
- Assa A, Butcher J, Li J, Elkadri A, Sherman PM, Muise AM, et al. Mucosa-associated ileal microbiota in new-onset pediatric Crohn's disease. Inflammatory Bowel Diseases 2016;22(7):1533-9. - PubMed
Austin 2014
-
- Austin M, Mellow M, Tierney WM. Fecal microbiota transplantation in the treatment of Clostridium difficile infections. American Journal of Medicine 2014;127(6):479-83. - PubMed
Auzoux 2017
-
- Auzoux J, Boschetti G, Lahlou W, Aubourg A, Girault A, Lecomte T, et al. Assessment of mucosal healing in inflammatory bowel disease. Journal of Clinical Gastroenterology and Hepatology 2017;1:1-8.
Bahnam 2023
-
- Bahnam P, Hanzel J, Ma C, Zou L, Narula N, Singh S, et al. Most placebo-controlled trials in inflammatory bowel disease were underpowered because of overestimated drug efficacy rates: results from a systematic review of induction studies. Journal of Crohn's and Colitis 2023;17(3):404-17. - PubMed
Bejaoui 2015
-
- Bejaoui M, Sokol H, Marteau P. Targeting the microbiome in inflammatory bowel disease: critical evaluation of current concepts and moving to new horizons. Digestive Diseases 2015;33(Suppl 1):105-12. - PubMed
Blanton 2016
Caldeira 2020
Cammarota 2015
-
- Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Alimentary Pharmacology and Therapeutics 2015;41(9):835-43. - PubMed
Cammarota 2017
Carding 2017
Carlson 2020
-
- Carlson PE Jr. Regulatory considerations for fecal microbiota transplantation products. Cell Host and Microbe 2020;27(2):173-5. - PubMed
Chassaing 2011
-
- Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140(6):1720-8. - PubMed
Chow 2011
Cleynen 2016
Colman 2014
Dahlhamer 2016
-
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged ≥18 years – United States, 2015. Morbidity and Mortality Weekly Report 2016;65(42):1166-9. - PubMed
Dave 2012
Davidovics 2019
-
- Davidovics ZH, Michail S, Nicholson MR, Kociolek LK, Pai N, Hansen R, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection and other conditions in children: a Joint Position Paper From the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2019;68(1):130-43. - PMC - PubMed
De Preter 2012
-
- De Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F, et al. Decreased mucosal sulfide detoxification is related to an impaired butyrate oxidation in ulcerative colitis. Inflammatory Bowel Diseases 2012;18(12):2371-80. - PubMed
Dulai 2015
Dumville 2006
FDA 2020a
-
- US Food and Drug Administration. Fecal microbiota for transplantation: safety alert – risk of serious adverse events likely due to transmission of pathogenic organisms. www.fda.gov/safety/medical-product-safety-information/fecal-microbiota-t... (accessed 1 April 2023).
FDA 2020b
-
- US Food and Drug Administration. Safety alert regarding use of fecal microbiota for transplantation and additional safety protections pertaining to SARS-CoV-2 and COVID-19. www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safet... (accessed 1 April 2023).
FDA 2022
-
- US Food and Drug Administration. Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. www.fda.gov/regulatory-information/search-fda-guidance-documents/enforce... (accessed 1 April 2023).
Feakins 2013
-
- Feakins RM, British Society of Gastroenterology. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. Journal of Clinical Pathology 2013;66(12):1005-26. - PubMed
Fehily 2021
-
- Fehily SR, Basnayake C, Wright EK, Kamm MA. Fecal microbiota transplantation therapy in Crohn's disease: systematic review. Journal of Gastroenterology and Hepatology 2021;36(10):2672-86. - PubMed
Feuerstadt 2022
-
- Feuerstadt P, Louie TJ, Lashner B, Wang EE, Diao L, Bryant JA, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. New England Journal of Medicine 2022;386(3):220-9. - PubMed
Fuentes 2017
GBD 2020
-
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterology and Hepatology 2020;5(1):17-30. - PMC - PubMed
Ghebre 2014
Gordon 2021
Gupta 2011
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hozo 2005
Iheozor‐Ejiofor 2020
Kassam 2013
-
- Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. American Journal of Gastroenterology 2013;108(4):500-8. - PubMed
Kaur 2020
Kelly 2014
Kelly 2015
Kelly 2016
Kelly 2021
-
- Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections [published correction appears in American Journal of Gastroenterology 2022 Feb 1;117(2):358]. American Journal of Gastroenterology 2021;116(6):1124-47. - PubMed
Khoruts 2016
Kostic 2014
Kunde 2013
-
- Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. Journal of Pediatric Gastroenterology and Nutrition 2013;56(6):597-601. - PubMed
Lam 2022
-
- Lam S, Bai X, Shkoporov AN, Park H, Wu X, Lan P, et al. Roles of the gut virome and mycobiome in faecal microbiota transplantation. Lancet Gastroenterology and Hepatology 2022;7(5):472-84. - PubMed
Lee 2016
-
- Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2016;315(2):142-9. - PubMed
Leffler 2015
-
- Leffler DA, Lamont JT. Clostridium difficile infection. New England Journal of Medicine 2015;372(16):1539-48. - PubMed
Link 2016
-
- Link A, Lachmund T, Schulz C, Weigt J, Malfertheiner P. Endoscopic peroral jejunal fecal microbiota transplantation. Digestive and Liver Disease 2016;48(11):1336-9. - PubMed
McDonald 2018
-
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile Infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical Infectious Diseases 2018;66(7):987-94. - PubMed
Mehta 2016
-
- Mehta F. Report: economic implications of inflammatory bowel disease and its management. American Journal of Managed Care 2016;22(Suppl 3):s51-60. - PubMed
Molodecky 2012
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46-e30. - PubMed
Moore 2014
-
- Moore T, Rodriguez A, Bakken JS. Fecal microbiota transplantation: a practical update for the infectious disease specialist. Clinical Infectious Diseases 2014;58(4):541-5. - PubMed
Morgan 2012
Mowat 2011
-
- Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60(5):571-607. - PubMed
Nagao‐Kitamoto 2016
Nageswaran 2022
Nguyen 2019
Nooruddin 2020
Osman 2016
-
- Osman M, Stoltzner Z, O'Brien K, Ling K, Koelsch E, Dubois N, et al. Donor efficacy in fecal microbiota transplantation for recurrent Clostridium difficile: evidence from a 1,999-patient cohort. Open Forum Infectious Diseases 2016;3(Suppl 1):841.
Owens 2013
-
- Owens C, Broussard E, Surawicz C. Fecal microbiota transplantation and donor standardization. Trends in Microbiology 2013;21(9):443-5. - PubMed
Paramsothy 2017
-
- Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. Journal of Crohn's and Colitis 2017;11(10):1180-99. - PubMed
Petersen 2014
Peyrin‐Biroulet 2011
-
- Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Results from the 2nd Scientific Workshop of the ECCO. I: impact of mucosal healing on the course of inflammatory bowel disease. Journal of Crohn's and Colitis 2011;5(5):477-83. - PubMed
Rapozo 2017
RevMan Web 2023 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.28.1. The Cochrane Collaboration, 2023. Available from www.revman.cochrane.org.
Ridaura 2013
Rode 2021
-
- Rode AA, Chehri M, Krogsgaard LR, Heno KK, Svendsen AT, Ribberholt I, et al. Randomised clinical trial: a 12-strain bacterial mixture versus faecal microbiota transplantation versus vancomycin for recurrent Clostridioides difficile infections. Alimentary Pharmacology and Therapeutics 2021;53(9):999-1009. - PubMed
Saha 2021
-
- Saha S, Mara K, Pardi DS, Khanna S. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology 2021;160(6):1961-9. - PubMed
Schulberg 2016
-
- Schulberg J, De Cruz P. Characterisation and therapeutic manipulation of the gut microbiome in inflammatory bowel disease. Internal Medicine Journal 2016;46(3):266-73. - PubMed
Shi 2016
Solari 2014
-
- Solari PR, Fairchild PG, Noa LJ, Wallace MR. Tempered enthusiasm for fecal transplant. Clinical Infectious Diseases 2014;59(2):319. - PubMed
Sun 2016
van Nood 2013
-
- Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. New England Journal of Medicine 2013;368(5):407-15. - PubMed
Vindigni 2016
-
- Vindigni SM, Zisman TL, Suskind DL, Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therapeutic Advances in Gastroenterology 2016;9(4):606-25. - PMC - PubMed
Weintraub 2014
Witherden 2017
Youngster 2014
-
- Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clinical Infectious Diseases 2014;58(11):1515-22. - PMC - PubMed
Zhao 2020
-
- Zhao HL, Chen SZ, Xu HM, Zhou YL, He J, Huang HL, et al. Efficacy and safety of fecal microbiota transplantation for treating patients with ulcerative colitis: a systematic review and meta-analysis. Journal of Digestive Diseases 2020;21(10):534-48. - PubMed
References to other published versions of this review
Imdad 2017
Imdad 2018
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

