YY1 complex in M2 macrophage promotes prostate cancer progression by upregulating IL-6
- PMID: 37094986
- PMCID: PMC10152059
- DOI: 10.1136/jitc-2022-006020
YY1 complex in M2 macrophage promotes prostate cancer progression by upregulating IL-6
Abstract
Background: Tumor-associated macrophages are mainly polarized into the M2 phenotype, remodeling the tumor microenvironment and promoting tumor progression by secreting various cytokines.
Methods: Tissue microarray consisting of prostate cancer (PCa), normal prostate, and lymph node metastatic samples from patients with PCa were stained with Yin Yang 1 (YY1) and CD163. Transgenic mice overexpressing YY1 were constructed to observe PCa tumorigenesis. Furthermore, in vivo and in vitro experiments, including CRISPR-Cas9 knock-out, RNA sequencing, chromatin immunoprecipitation (ChIP) sequencing, and liquid-liquid phase separation (LLPS) assays, were performed to investigate the role and mechanism of YY1 in M2 macrophages and PCa tumor microenvironment.
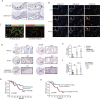
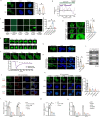
Results: YY1 was highly expressed in M2 macrophages in PCa and was associated with poorer clinical outcomes. The proportion of tumor-infiltrated M2 macrophages increased in transgenic mice overexpressing YY1. In contrast, the proliferation and activity of anti-tumoral T lymphocytes were suppressed. Treatment targeting YY1 on M2 macrophages using an M2-targeting peptide-modified liposome carrier suppressed PCa cell lung metastasis and generated synergistic anti-tumoral effects with PD-1 blockade. IL-4/STAT6 pathway regulated YY1, and YY1 increased the macrophage-induced PCa progression by upregulating IL-6. Furthermore, by conducting H3K27ac-ChIP-seq in M2 macrophages and THP-1, we found that thousands of enhancers were gained during M2 macrophage polarization, and these M2-specific enhancers were enriched in YY1 ChIP-seq signals. In addition, an M2-specific IL-6 enhancer upregulated IL-6 expression through long-range chromatin interaction with IL-6 promoter in M2 macrophages. During M2 macrophage polarization, YY1 formed an LLPS, in which p300, p65, and CEBPB acted as transcriptional cofactors.
Conclusions: Phase separation of the YY1 complex in M2 macrophages upregulated IL-6 by promoting IL-6 enhancer-promoter interactions, thereby increasing PCa progression.
Keywords: cytokines; macrophages; prostatic neoplasms; tumor microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
