Metabolomic differentiation of benign vs malignant pulmonary nodules with high specificity via high-resolution mass spectrometry analysis of patient sera
- PMID: 37095081
- PMCID: PMC10126054
- DOI: 10.1038/s41467-023-37875-1
Metabolomic differentiation of benign vs malignant pulmonary nodules with high specificity via high-resolution mass spectrometry analysis of patient sera
Abstract
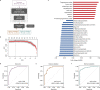
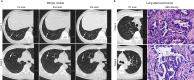
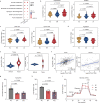
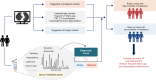
Differential diagnosis of pulmonary nodules detected by computed tomography (CT) remains a challenge in clinical practice. Here, we characterize the global metabolomes of 480 serum samples including healthy controls, benign pulmonary nodules, and stage I lung adenocarcinoma. The adenocarcinoma demonstrates a distinct metabolomic signature, whereas benign nodules and healthy controls share major similarities in metabolomic profiles. A panel of 27 metabolites is identified in the discovery cohort (n = 306) to distinguish between benign and malignant nodules. The discriminant model achieves an AUC of 0.915 and 0.945 in the internal validation (n = 104) and external validation cohort (n = 111), respectively. Pathway analysis reveals elevation in glycolytic metabolites associated with decreased tryptophan in serum of lung adenocarcinoma vs benign nodules and healthy controls, and demonstrates that uptake of tryptophan promotes glycolysis in lung cancer cells. Our study highlights the value of the serum metabolite biomarkers in risk assessment of pulmonary nodules detected by CT screening.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

