Allogenic and autologous anti-CD7 CAR-T cell therapies in relapsed or refractory T-cell malignancies
- PMID: 37095094
- PMCID: PMC10125858
- DOI: 10.1038/s41408-023-00822-w
Allogenic and autologous anti-CD7 CAR-T cell therapies in relapsed or refractory T-cell malignancies
Abstract
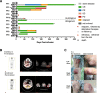
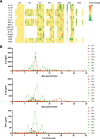
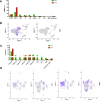
Chimeric antigen receptor-T (CAR-T) therapy remains to be investigated in T-cell malignancies. CD7 is an ideal target for T-cell malignancies but is also expressed on normal T cells, which may cause CAR-T cell fratricide. Donor-derived anti-CD7 CAR-T cells using endoplasmic reticulum retention have shown efficacy in patients with T-cell acute lymphoblastic leukemia (ALL). Here we launched a phase I trial to explore differences between autologous and allogeneic anti-CD7 CAR-T therapies in T-cell ALL and lymphoma. Ten patients were treated and 5 received autologous CAR-T therapies. No dose-limiting toxicity or neurotoxicity was observed. Grade 1-2 cytokine release syndrome occurred in 7 patients, and grade 3 in 1 patient. Grade 1-2 graft-versus-host diseases were observed in 2 patients. Seven patients had bone marrow infiltration, and 100% of them achieved complete remission with negative minimal residual disease within one month. Two-fifths of patients achieved extramedullary or extranodular remission. The median follow-up was 6 (range, 2.7-14) months and bridging transplantation was not administrated. Patients treated with allogeneic CAR-T cells had higher remission rate, less recurrence and more durable CAR-T survival than those receiving autologous products. Allogeneic CAR-T cells appeared to be a better option for patients with T-cell malignancies.
© 2023. The Author(s).
Conflict of interest statement
AHC is a founding member of Shanghai YaKe Biotechnology Ltd, a biotechnology company focusing on research and development of tumor cellular immunotherapy. YLZ is employed in Shanghai YaKe Biotechnology Ltd, DW is employed in Beijing GoBroad Hospital Management Co. Ltd and the other authors have declared that no conflict of interest exists.
Figures

References
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. doi: 10.1016/S0140-6736(14)61403-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

