The biophysical property of the limbal niche maintains stemness through YAP
- PMID: 37095157
- PMCID: PMC10244376
- DOI: 10.1038/s41418-023-01156-7
The biophysical property of the limbal niche maintains stemness through YAP
Abstract
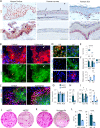
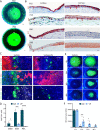
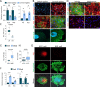
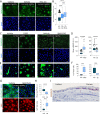
The cell fate decisions of stem cells (SCs) largely depend on signals from their microenvironment (niche). However, very little is known about how biochemical niche cues control cell behavior in vivo. To address this question, we focused on the corneal epithelial SC model in which the SC niche, known as the limbus, is spatially segregated from the differentiation compartment. We report that the unique biomechanical property of the limbus supports the nuclear localization and function of Yes-associated protein (YAP), a putative mediator of the mechanotransduction pathway. Perturbation of tissue stiffness or YAP activity affects SC function as well as tissue integrity under homeostasis and significantly inhibited the regeneration of the SC population following SC depletion. In vitro experiments revealed that substrates with the rigidity of the corneal differentiation compartment inhibit nuclear YAP localization and induce differentiation, a mechanism that is mediated by the TGFβ-SMAD2/3 pathway. Taken together, these results indicate that SC sense biomechanical niche signals and that manipulation of mechano-sensory machinery or its downstream biochemical output may bear fruits in SC expansion for regenerative therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

