A shared neural basis underlying psychiatric comorbidity
- PMID: 37095248
- PMCID: PMC10202801
- DOI: 10.1038/s41591-023-02317-4
A shared neural basis underlying psychiatric comorbidity
Erratum in
-
Author Correction: A shared neural basis underlying psychiatric comorbidity.Nat Med. 2023 Sep;29(9):2375. doi: 10.1038/s41591-023-02512-3. Nat Med. 2023. PMID: 37558759 Free PMC article. No abstract available.
Abstract
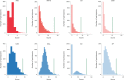
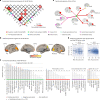
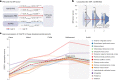
Recent studies proposed a general psychopathology factor underlying common comorbidities among psychiatric disorders. However, its neurobiological mechanisms and generalizability remain elusive. In this study, we used a large longitudinal neuroimaging cohort from adolescence to young adulthood (IMAGEN) to define a neuropsychopathological (NP) factor across externalizing and internalizing symptoms using multitask connectomes. We demonstrate that this NP factor might represent a unified, genetically determined, delayed development of the prefrontal cortex that further leads to poor executive function. We also show this NP factor to be reproducible in multiple developmental periods, from preadolescence to early adulthood, and generalizable to the resting-state connectome and clinical samples (the ADHD-200 Sample and the Stratify Project). In conclusion, we identify a reproducible and general neural basis underlying symptoms of multiple mental health disorders, bridging multidimensional evidence from behavioral, neuroimaging and genetic substrates. These findings may help to develop new therapeutic interventions for psychiatric comorbidities.
© 2023. The Author(s).
Conflict of interest statement
T.B. served in an advisory or consultancy role for Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH and Shire. He received conference support or speaker’s fee from Lilly, Medice, Novartis and Shire. He has been involved in clinical trials conducted by Shire and Viforpharma. He received royalties from Hogrefe, Kohlhammer, CIP Medien and Oxford University Press. The present work is unrelated to the above grants and relationships. G.J.B. received honoraria from General Electric Healthcare for teaching scanner programming courses. All other authors declare no competing interests.
Figures




References
-
- van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur. J. Gen. Pract. 1996;2:65–70. doi: 10.3109/13814789609162146. - DOI
Publication types
MeSH terms
Grants and funding
- MR/R00465X/1/MRC_/Medical Research Council/United Kingdom
- MRF-058-0009-RG-DESR-C0759/MRC_/Medical Research Council/United Kingdom
- R01 MH085772/MH/NIMH NIH HHS/United States
- U54 EB020403/EB/NIBIB NIH HHS/United States
- MR/S020306/1/MRC_/Medical Research Council/United Kingdom
- MR/W002418/1/MRC_/Medical Research Council/United Kingdom
- R01 DA049238/DA/NIDA NIH HHS/United States
- MRF-058-0004-RG-DESRI/MRF_/MRF_/United Kingdom
- MR/N000390/1 /MRC_/Medical Research Council/United Kingdom
- MRF-058-0009-RG-DESR-C0759/MRF_/MRF_/United Kingdom
- R56 AG058854/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

