Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants
- PMID: 37095249
- PMCID: PMC10202809
- DOI: 10.1038/s41591-023-02316-5
Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants
Erratum in
-
Author Correction: Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants.Nat Med. 2024 Jun;30(6):1785. doi: 10.1038/s41591-024-03006-6. Nat Med. 2024. PMID: 38649782 Free PMC article. No abstract available.
Abstract
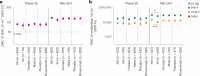
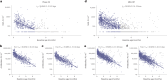
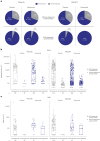
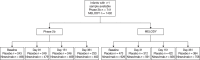
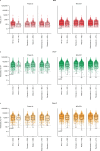
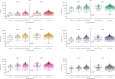
Nirsevimab is an extended half-life monoclonal antibody specific for the prefusion conformation of the respiratory syncytial virus (RSV) F protein, which has been studied in preterm and full-term infants in the phase 2b and phase 3 MELODY trials. We analyzed serum samples collected from 2,143 infants during these studies to characterize baseline levels of RSV-specific immunoglobulin G antibodies and neutralizing antibodies (NAbs), duration of RSV NAb levels following nirsevimab administration, the risk of RSV exposure during the first year of life and the infant's adaptive immune response to RSV following nirsevimab administration. Baseline RSV antibody levels varied widely; consistent with reports that maternal antibodies are transferred late in the third trimester, preterm infants had lower baseline RSV antibody levels than full-term infants. Nirsevimab recipients had RSV NAb levels >140-fold higher than baseline at day 31 and remained >50-fold higher at day 151 and >7-fold higher at day 361. Similar seroresponse rates to the postfusion form of RSV F protein in nirsevimab recipients (68-69%) compared with placebo recipients (63-70%; not statistically significant) suggest that while nirsevimab protects from RSV disease, it still allows an active immune response. In summary, nirsevimab provided sustained, high levels of NAb throughout an infant's first RSV season and prevented RSV disease while allowing the development of an immune response to RSV.
© 2023. The Author(s).
Conflict of interest statement
D.W., Y.C., A.A.A., B.S.N., U.W.-H., T.Z., M.E.A., A.L., T.V. and M.T.E. are employees of and may hold stock in AstraZeneca. Y.Y. is a former employee of AstraZeneca.
Figures







Comment in
-
Game over for RSV?Sci Immunol. 2023 Jun 8;8(84):eadi8764. doi: 10.1126/sciimmunol.adi8764. Epub 2023 Jun 2. Sci Immunol. 2023. PMID: 37276355 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

