Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer's disease: a phase 1b, randomized, placebo-controlled trial
- PMID: 37095250
- PMCID: PMC10287562
- DOI: 10.1038/s41591-023-02326-3
Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer's disease: a phase 1b, randomized, placebo-controlled trial
Erratum in
-
Author Correction: Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer's disease: a phase 1b, randomized, placebo-controlled trial.Nat Med. 2024 Jan;30(1):304. doi: 10.1038/s41591-023-02639-3. Nat Med. 2024. PMID: 37845513 Free PMC article. No abstract available.
Abstract
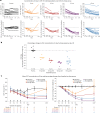
Tau plays a key role in Alzheimer's disease (AD) pathophysiology, and accumulating evidence suggests that lowering tau may reduce this pathology. We sought to inhibit MAPT expression with a tau-targeting antisense oligonucleotide (MAPTRx) and reduce tau levels in patients with mild AD. A randomized, double-blind, placebo-controlled, multiple-ascending dose phase 1b trial evaluated the safety, pharmacokinetics and target engagement of MAPTRx. Four ascending dose cohorts were enrolled sequentially and randomized 3:1 to intrathecal bolus administrations of MAPTRx or placebo every 4 or 12 weeks during the 13-week treatment period, followed by a 23 week post-treatment period. The primary endpoint was safety. The secondary endpoint was MAPTRx pharmacokinetics in cerebrospinal fluid (CSF). The prespecified key exploratory outcome was CSF total-tau protein concentration. Forty-six patients enrolled in the trial, of whom 34 were randomized to MAPTRx and 12 to placebo. Adverse events were reported in 94% of MAPTRx-treated patients and 75% of placebo-treated patients; all were mild or moderate. No serious adverse events were reported in MAPTRx-treated patients. Dose-dependent reduction in the CSF total-tau concentration was observed with greater than 50% mean reduction from baseline at 24 weeks post-last dose in the 60 mg (four doses) and 115 mg (two doses) MAPTRx groups. Clinicaltrials.gov registration number: NCT03186989 .
© 2023. The Author(s).
Conflict of interest statement
C.J.M. reports advisory board membership for Roche, Lilly, Biogen and Ionis; research grants from Biogen; seminar chair for Biogen; chair of data safety monitoring board for AD trial led by Imperial College; supported by the NIHR Biomedical Research Centre dementia subtheme at UCLH. M.J. reports advisory board membership for Biogen Sweden AB and BioArctic AB. S.D. reports salary support from the Fonds de recherche du Québec – Santé; consultancies from Biogen, Wave Life Sciences, AZTherapies and Janssen Pharmaceuticals; advisory board membership and/or speaker fees from Eisai, Biogen, Sunovion, Innodem Neurosciences, HealthTech Connex and QurAlis; cofounder of AFX Medical Inc. E.G.B.V. reports consultancies from New Amsterdam Pharma, Treeway, ReMynd, Vivoryon, Biogen, Vigil Neuroscience, ImmunoBrain Checkpoint and Brainstorm Therapeutics. PI of studies with AC immune, CogRX therapeutics, New Amsterdam Pharma, Janssen, UCB, Roche, GreenValley, Vivoryon, ImmunoBrain, Alector and Alzheon and sub-I from DIAN-TU, Alzheon, Eli Lilly, Cortexyme, Biogen en Fuij Film Toyama. L.M., K.M.M., C.Y., D.L., D.A.N., R.C., C.F.B., C.J. and R.M.L. are employees of, and hold stock in, Ionis. D.L.G., E.H. and E.R. are employees of, and hold stock in, Biogen. The remaining authors declare no competing interests.
Figures



Comment in
-
Path from discovery to recovery: therapeutic and diagnostic advances in Alzheimer's dementia.J Neurol. 2023 Aug;270(8):4151-4153. doi: 10.1007/s00415-023-11840-w. Epub 2023 Jul 3. J Neurol. 2023. PMID: 37394517 Free PMC article. No abstract available.
References
-
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement.17, 327–406 (2021). - PubMed
-
- 2020 Alzheimer’s disease facts and figures. Alzheimers Dement.10.1002/alz.12068 (2020).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

