A pilot study comparing the efficacy of autologous cultured fibroblast injections with hyaluronic acid fillers for treating nasolabial folds
- PMID: 37095274
- PMCID: PMC10126053
- DOI: 10.1038/s41598-023-33786-9
A pilot study comparing the efficacy of autologous cultured fibroblast injections with hyaluronic acid fillers for treating nasolabial folds
Abstract
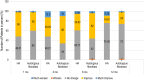
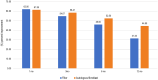
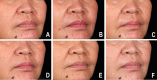
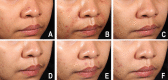
Autologous cultured fibroblast injections for soft tissue augmentation are a potential alternative to other filler materials. No studies have compared autologous fibroblast injections and hyaluronic acid (HA) fillers for treating nasolabial folds (NLFs). To compare the efficacies and safeties of autologous cultured fibroblast injections and HA fillers for treating NLFs. This prospective, evaluator-blinded, pilot study enrolled 60 Thai female adult patients diagnosed with moderate to severe NLFs. They were randomized to receive either 3 treatments of autologous fibroblasts at 2-week intervals or 1 treatment with HA fillers. The primary outcome was the clinical improvement of the NLFs graded by 2 blinded dermatologists immediately after injection and at 1-, 3-, 6-, and 12-month follow-ups. Objective measurement of the NLF volume was evaluated. Patient self-assessment scores, pain scores, and adverse reactions were recorded. Of the 60 patients, 55 (91.7%) completed the study protocol. The NLF volumes improved significantly in the autologous fibroblast group at all follow-ups relative to baseline (P = 0.000, 0.004, 0.000, 0.000, and 0.003). The patients in the autologous fibroblast group rated more noticeable NLF improvements than those in the HA filler group (3-month follow-up, 58.41% vs. 54.67%; 6-month follow-up, 52.50% vs. 46%; 12-month follow-up, 44.55% vs. 31.33%). No serious adverse reactions were recorded. Autologous fibroblast injections are safe and effective for treating NLFs. These injections also promise sustained growth of living cells, possibly leading to a greater persistence than shown by other fillers.
© 2023. The Author(s).
Conflict of interest statement
Financial competing interests. Saowalak Thanachaipiwat, Uraiwan Panich, and Rungsima Wanitphakdeedecha are the inventors of autologous fibroblast culture and preparation. Petty patent application has been filed by Institute for Technology and Innovation Management (INT), Mahidol University at the Department of Intellectual Property since March 10, 2022 with application number of 2203000625. The status of the application is now under reviewed. All other authors declare that they have no conflicts of interest related to any aspect of this research.
Figures
Similar articles
-
A 52-week follow-up, multi-center, randomized, double-blinded comparison of efficacy and safety of two hyaluronic acid fillers for the treatment of moderate-to-severe nasolabial folds in Chinese population.J Dermatolog Treat. 2024 Dec;35(1):2378165. doi: 10.1080/09546634.2024.2378165. Epub 2024 Jul 14. J Dermatolog Treat. 2024. PMID: 39004426 Clinical Trial.
-
Efficacy and safety of two hyaluronic acid fillers with different injection depths for the correction of moderate-to-severe nasolabial folds: A 52-week, prospective, randomized, double-blinded study in a Chinese population.J Cosmet Dermatol. 2022 Mar;21(3):940-948. doi: 10.1111/jocd.14744. Epub 2022 Jan 12. J Cosmet Dermatol. 2022. PMID: 35020250 Clinical Trial.
-
Clinical efficacy and safety of polynucleotides highly purified technology (PN-HPT®) and cross-linked hyaluronic acid for moderate to severe nasolabial folds: A prospective, randomized, exploratory study.J Cosmet Dermatol. 2023 Jan;22(1):146-155. doi: 10.1111/jocd.15064. Epub 2022 May 26. J Cosmet Dermatol. 2023. PMID: 35531796 Free PMC article. Clinical Trial.
-
Monophasic and Biphasic Hyaluronic Acid Fillers for Esthetic Correction of Nasolabial Folds: A Meta-Analysis of Randomized Controlled Trials.Aesthetic Plast Surg. 2022 Jun;46(3):1407-1422. doi: 10.1007/s00266-021-02729-y. Epub 2022 Jan 23. Aesthetic Plast Surg. 2022. PMID: 35066619 Review.
-
Monophasic versus biphasic hyaluronic acid filler for correcting nasolabial folds: a systematic review and meta-analysis.J Cosmet Dermatol. 2022 Feb;21(2):627-635. doi: 10.1111/jocd.14632. Epub 2021 Nov 24. J Cosmet Dermatol. 2022. PMID: 34817919
Cited by
-
Skin Rejuvenation Using Autologous Cultured Fibroblast Grafting.Cureus. 2024 Dec 9;16(12):e75405. doi: 10.7759/cureus.75405. eCollection 2024 Dec. Cureus. 2024. PMID: 39781128 Free PMC article.
-
A comprehensive evaluation of dermal fibroblast therapy in clinical trials for treating skin disorders and cosmetic applications: a scoping review.Stem Cell Res Ther. 2024 Sep 20;15(1):318. doi: 10.1186/s13287-024-03892-0. Stem Cell Res Ther. 2024. PMID: 39304949 Free PMC article.
-
Post Hoc Non-inferiority Analysis of the Efficacy of Poly-L-Lactic Acid Filler (Gana V versus Sculptra) Injection for Correction of the Nasolabial Fold: A Double-Blind, Randomized, Split-Face Controlled Trial.Aesthetic Plast Surg. 2024 Dec 28. doi: 10.1007/s00266-024-04629-3. Online ahead of print. Aesthetic Plast Surg. 2024. PMID: 39733049 No abstract available.
References
-
- Puizina-Ivić N. Skin aging. Acta Dermatovenerol. Alp Pannonica Adriat. 2008;17(2):47–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

