Perennial malaria chemoprevention with and without malaria vaccination to reduce malaria burden in young children: a modelling analysis
- PMID: 37095480
- PMCID: PMC10124689
- DOI: 10.1186/s12936-023-04564-9
Perennial malaria chemoprevention with and without malaria vaccination to reduce malaria burden in young children: a modelling analysis
Abstract
Background: A recent WHO recommendation for perennial malaria chemoprevention (PMC) encourages countries to adapt dose timing and number to local conditions. However, knowledge gaps on the epidemiological impact of PMC and possible combination with the malaria vaccine RTS,S hinder informed policy decisions in countries where malaria burden in young children remains high.
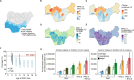
Methods: The EMOD malaria model was used to predict the impact of PMC with and without RTS,S on clinical and severe malaria cases in children under the age of two years (U2). PMC and RTS,S effect sizes were fit to trial data. PMC was simulated with three to seven doses (PMC-3-7) before the age of eighteen months and RTS,S with three doses, shown to be effective at nine months. Simulations were run for transmission intensities of one to 128 infectious bites per person per year, corresponding to incidences of < 1 to 5500 cases per 1000 population U2. Intervention coverage was either set to 80% or based on 2018 household survey data for Southern Nigeria as a sample use case. The protective efficacy (PE) for clinical and severe cases in children U2 was calculated in comparison to no PMC and no RTS,S.
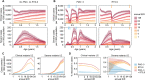
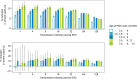
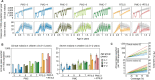
Results: The projected impact of PMC or RTS,S was greater at moderate to high transmission than at low or very high transmission. Across the simulated transmission levels, PE estimates of PMC-3 at 80% coverage ranged from 5.7 to 8.8% for clinical, and from 6.1 to 13.6% for severe malaria (PE of RTS,S 10-32% and 24.6-27.5% for clinical and severe malaria, respectively. In children U2, PMC with seven doses nearly averted as many cases as RTS,S, while the combination of both was more impactful than either intervention alone. When operational coverage, as seen in Southern Nigeria, increased to a hypothetical target of 80%, cases were reduced beyond the relative increase in coverage.
Conclusions: PMC can substantially reduce clinical and severe cases in the first two years of life in areas with high malaria burden and perennial transmission. A better understanding of the malaria risk profile by age in early childhood and on feasible coverage by age, is needed for selecting an appropriate PMC schedule in a given setting.
Keywords: EMOD; Malaria chemoprevention; Malaria modeling; Malaria prevention; Malaria vaccine; Mathematical modeling; Nigeria; PMC; RTS,S.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Seasonal use case for the RTS,S/AS01 malaria vaccine: a mathematical modelling study.Lancet Glob Health. 2022 Dec;10(12):e1782-e1792. doi: 10.1016/S2214-109X(22)00416-8. Lancet Glob Health. 2022. PMID: 36400084 Clinical Trial.
-
Seasonal vaccination with RTS,S/AS01E vaccine with or without seasonal malaria chemoprevention in children up to the age of 5 years in Burkina Faso and Mali: a double-blind, randomised, controlled, phase 3 trial.Lancet Infect Dis. 2024 Jan;24(1):75-86. doi: 10.1016/S1473-3099(23)00368-7. Epub 2023 Aug 22. Lancet Infect Dis. 2024. PMID: 37625434 Clinical Trial.
-
The duration of protection against clinical malaria provided by the combination of seasonal RTS,S/AS01E vaccination and seasonal malaria chemoprevention versus either intervention given alone.BMC Med. 2022 Oct 7;20(1):352. doi: 10.1186/s12916-022-02536-5. BMC Med. 2022. PMID: 36203149 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
RTS,S/AS01 malaria vaccine (Mosquirix®): a profile of its use.Drugs Ther Perspect. 2022;38(9):373-381. doi: 10.1007/s40267-022-00937-3. Epub 2022 Sep 7. Drugs Ther Perspect. 2022. PMID: 36093265 Free PMC article. Review.
Cited by
-
A roadmap of priority evidence gaps for the co-implementation of malaria vaccines and perennial malaria chemoprevention.Malar J. 2025 Apr 17;24(1):126. doi: 10.1186/s12936-025-05347-0. Malar J. 2025. PMID: 40247263 Free PMC article.
-
Effectiveness of malaria chemoprevention in the first two years of life in Cameroon and Côte d'Ivoire compared to standard of care: study protocol for a population-based prospective cohort impact evaluation study.BMC Public Health. 2024 Sep 6;24(1):2430. doi: 10.1186/s12889-024-19887-8. BMC Public Health. 2024. PMID: 39243075 Free PMC article.
-
Public health impact of current and proposed age-expanded perennial malaria chemoprevention: a modelling study.Sci Rep. 2025 Mar 26;15(1):10488. doi: 10.1038/s41598-025-93623-z. Sci Rep. 2025. PMID: 40140443 Free PMC article.
-
Malaria prevention in children: an update.Curr Opin Pediatr. 2024 Apr 1;36(2):164-170. doi: 10.1097/MOP.0000000000001332. Epub 2024 Jan 22. Curr Opin Pediatr. 2024. PMID: 38299986 Free PMC article. Review.
-
Enhancing malaria elimination in high-transmission settings: the synergy of concurrent vector control and chemotherapy.Malar J. 2025 Apr 1;24(1):105. doi: 10.1186/s12936-025-05339-0. Malar J. 2025. PMID: 40170076 Free PMC article. Review.
References
-
- WHO . World malaria report 2022. Geneva: World Health Organization; 2022.
-
- WHO . World malaria report 2021. Geneva: World Health Organization; 2021.
-
- Lahuerta M, Sutton R, Mansaray A, Eleeza O, Gleason B, Akinjeji A, et al. Evaluation of health system readiness and coverage of intermittent preventive treatment of malaria in infants (IPTi) in Kambia district to inform national scale-up in Sierra Leone. Malar J. 2021;20:74. doi: 10.1186/s12936-021-03615-3. - DOI - PMC - PubMed
-
- WHO . Guidelines for malaria. Geneva: World Health Organization; 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

