Experimental studies from shake flasks to 3 L stirred tank bioreactor of nutrients and oxygen supply conditions to improve the growth of the avian cell line DuckCelt®-T17
- PMID: 37095522
- PMCID: PMC10127095
- DOI: 10.1186/s13036-023-00349-5
Experimental studies from shake flasks to 3 L stirred tank bioreactor of nutrients and oxygen supply conditions to improve the growth of the avian cell line DuckCelt®-T17
Abstract
Background: To produce viral vaccines, avian cell lines are interesting alternatives to replace the egg-derived processes for viruses that do not grow well on mammalian cells. The avian suspension cell line DuckCelt®-T17 was previously studied and investigated to produce a live attenuated metapneumovirus (hMPV)/respiratory syncytial virus (RSV) and influenza virus vaccines. However, a better understanding of its culture process is necessary for an efficient production of viral particles in bioreactors.
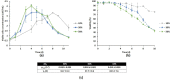
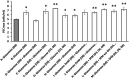
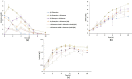
Results: The growth and metabolic requirements of the avian cell line DuckCelt®-T17 were investigated to improve its cultivation parameters. Several nutrient supplementation strategies were studied in shake flasks highlighting the interest of (i) replacing L-glutamine by glutamax as main nutrient or (ii) adding these two nutrients in the serum-free growth medium in a fed-batch strategy. The scale-up in a 3 L bioreactor was successful for these types of strategies confirming their efficiencies in improving the cells' growth and viability. Moreover, a perfusion feasibility test allowed to achieve up to ~ 3 times the maximum number of viable cells obtained with the batch or fed-batch strategies. Finally, a strong oxygen supply - 50% dO2 - had a deleterious effect on DuckCelt®-T17 viability, certainly because of the greater hydrodynamic stress imposed.
Conclusions: The culture process using glutamax supplementation with a batch or a fed-batch strategy was successfully scaled-up to 3 L bioreactor. In addition, perfusion appeared as a very promising culture process for subsequent continuous virus harvesting.
Keywords: Cell growth; DuckCelt®-T17 avian cell line; Fed-batch culture; Glutamax; Nutrient supplementation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Influenza viruses production: Evaluation of a novel avian cell line DuckCelt®-T17.Vaccine. 2018 May 24;36(22):3101-3111. doi: 10.1016/j.vaccine.2017.03.102. Epub 2017 May 29. Vaccine. 2018. PMID: 28571695
-
Avian Cell Line DuckCelt®-T17 Is an Efficient Production System for Live-Attenuated Human Metapneumovirus Vaccine Candidate Metavac®.Vaccines (Basel). 2021 Oct 16;9(10):1190. doi: 10.3390/vaccines9101190. Vaccines (Basel). 2021. PMID: 34696298 Free PMC article.
-
High-cell-density cultivations to increase MVA virus production.Vaccine. 2018 May 24;36(22):3124-3133. doi: 10.1016/j.vaccine.2017.10.112. Epub 2018 Feb 9. Vaccine. 2018. PMID: 29433897 Free PMC article.
-
High cell density cultivation and recombinant protein production with Escherichia coli in a rocking-motion-type bioreactor.Microb Cell Fact. 2010 May 30;9:42. doi: 10.1186/1475-2859-9-42. Microb Cell Fact. 2010. PMID: 20509968 Free PMC article.
-
Benchtop Bioreactors in Mammalian Cell Culture: Overview and Guidelines.Methods Mol Biol. 2022;2436:1-15. doi: 10.1007/7651_2021_441. Methods Mol Biol. 2022. PMID: 34611816 Review.
Cited by
-
Innovations in cell culture-based influenza vaccine manufacturing - from static cultures to high cell density cultivations.Hum Vaccin Immunother. 2024 Dec 31;20(1):2373521. doi: 10.1080/21645515.2024.2373521. Epub 2024 Jul 15. Hum Vaccin Immunother. 2024. PMID: 39007904 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

