Submersion and hypoxia inhibit alveolar epithelial Na+ transport through ERK/NF-κB signaling pathway
- PMID: 37095538
- PMCID: PMC10127099
- DOI: 10.1186/s12931-023-02428-z
Submersion and hypoxia inhibit alveolar epithelial Na+ transport through ERK/NF-κB signaling pathway
Abstract
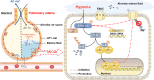
Background: Hypoxia is associated with many respiratory diseases, partly due to the accumulation of edema fluid and mucus on the surface of alveolar epithelial cell (AEC), which forms oxygen delivery barriers and is responsible for the disruption of ion transport. Epithelial sodium channel (ENaC) on the apical side of AEC plays a crucial role to maintain the electrochemical gradient of Na+ and water reabsorption, thus becomes the key point for edema fluid removal under hypoxia. Here we sought to explore the effects of hypoxia on ENaC expression and the further mechanism related, which may provide a possible treatment strategy in edema related pulmonary diseases.
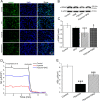
Methods: Excess volume of culture medium was added on the surface of AEC to simulate the hypoxic environment of alveoli in the state of pulmonary edema, supported by the evidence of increased hypoxia-inducible factor-1 expression. The protein/mRNA expressions of ENaC were detected, and extracellular signal-regulated kinase (ERK)/nuclear factor κB (NF-κB) inhibitor was applied to explore the detailed mechanism about the effects of hypoxia on epithelial ion transport in AEC. Meanwhile, mice were placed in chambers with normoxic or hypoxic (8%) condition for 24 h, respectively. The effects of hypoxia and NF-κB were assessed through alveolar fluid clearance and ENaC function by Ussing chamber assay.
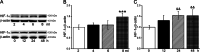
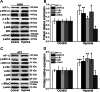
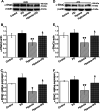
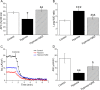
Results: Hypoxia (submersion culture mode) induced the reduction of protein/mRNA expression of ENaC, whereas increased the activation of ERK/NF-κB signaling pathway in parallel experiments using human A549 and mouse alveolar type 2 cells, respectively. Moreover, the inhibition of ERK (PD98059, 10 µM) alleviated the phosphorylation of IκB and p65, implying NF-κB as a downstream pathway involved with ERK regulation. Intriguingly, the expression of α-ENaC could be reversed by either ERK or NF-κB inhibitor (QNZ, 100 nM) under hypoxia. The alleviation of pulmonary edema was evidenced by the administration of NF-κB inhibitor, and enhancement of ENaC function was supported by recording amiloride-sensitive short-circuit currents.
Conclusions: The expression of ENaC was downregulated under hypoxia induced by submersion culture, which may be mediated by ERK/NF-κB signaling pathway.
Keywords: Alveolar epithelial cell; Epithelial sodium channel; Extracellular signal-regulated kinase; Hypoxia; Nuclear factor κB.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Mesenchymal stem cell-secreted KGF ameliorates acute lung injury via the Gab1/ERK/NF-κB signaling axis.Cell Mol Biol Lett. 2025 Jul 10;30(1):79. doi: 10.1186/s11658-025-00757-z. Cell Mol Biol Lett. 2025. PMID: 40640718 Free PMC article.
-
Hypoxia-induced inhibition of epithelial Na(+) channels in the lung. Role of Nedd4-2 and the ubiquitin-proteasome pathway.Am J Respir Cell Mol Biol. 2014 Mar;50(3):526-37. doi: 10.1165/rcmb.2012-0518OC. Am J Respir Cell Mol Biol. 2014. PMID: 24093724
-
Dexamethasone prevents transport inhibition by hypoxia in rat lung and alveolar epithelial cells by stimulating activity and expression of Na+-K+-ATPase and epithelial Na+ channels.Am J Physiol Lung Cell Mol Physiol. 2007 Nov;293(5):L1332-8. doi: 10.1152/ajplung.00338.2006. Epub 2007 Sep 14. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17873005
-
Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice.Cell Physiol Biochem. 2010;25(1):63-70. doi: 10.1159/000272051. Epub 2009 Dec 22. Cell Physiol Biochem. 2010. PMID: 20054145 Review.
-
Treatment of pulmonary edema by ENaC activators/stimulators.Curr Mol Pharmacol. 2013 Mar;6(1):13-27. doi: 10.2174/1874467211306010003. Curr Mol Pharmacol. 2013. PMID: 23547931 Review.
Cited by
-
Low-pressure exposure influences the development of HAPE.Open Life Sci. 2025 Apr 1;20(1):20221029. doi: 10.1515/biol-2022-1029. eCollection 2025. Open Life Sci. 2025. PMID: 40177420 Free PMC article.
-
Mesenchymal stem cell-secreted KGF ameliorates acute lung injury via the Gab1/ERK/NF-κB signaling axis.Cell Mol Biol Lett. 2025 Jul 10;30(1):79. doi: 10.1186/s11658-025-00757-z. Cell Mol Biol Lett. 2025. PMID: 40640718 Free PMC article.
References
-
- Peters DM, Vadász I, Wujak L, Wygrecka M, Olschewski A, Becker C, et al. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci U S A. 2014;111(3):E374–383. doi: 10.1073/pnas.1306798111. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

