Lassa fever vaccine candidates: A scoping review of vaccine clinical trials
- PMID: 37095630
- PMCID: PMC10247453
- DOI: 10.1111/tmi.13876
Lassa fever vaccine candidates: A scoping review of vaccine clinical trials
Abstract
Objective: Lassa fever (LF) is caused by a viral pathogen with pandemic potential. LF vaccines have the potential to prevent significant disease in individuals at risk of infection, but no such vaccine has been licensed or authorised for use thus far. We conducted a scoping review to identify and compare registered phase 1, 2 or 3 clinical trials of LF vaccine candidates, and appraise the current trajectory of LF vaccine development.
Method: We systematically searched 24 trial registries, PubMed, relevant conference abstracts and additional grey literature sources up to 27 October 2022. After extracting key details about each vaccine candidate and each eligible trial, we qualitatively synthesised the evidence.
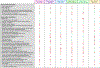
Results: We found that four LF vaccine candidates (INO-4500, MV-LASV, rVSV∆G-LASV-GPC, and EBS-LASV) have entered the clinical stage of assessment. Five phase 1 trials (all focused on healthy adults) and one phase 2 trial (involving a broader age group from 18 months to 70 years) evaluating one of these vaccines have been registered to date. Here, we describe the characteristics of each vaccine candidate and trial and compare them to WHO's target product profile for Lassa vaccines.
Conclusion: Though LF vaccine development is still in early stages, current progress towards a safe and effective vaccine is encouraging.
Keywords: LASV; clinical trial; priority pathogens; vaccine candidate; vaccine development; viral haemorrhagic fever.
© 2023 The Authors. Tropical Medicine & International Health Published by John Wiley & Sons Ltd.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

