AL(light chain)-amyloidogenesis by mesangial cells involves active participation of lysosomes: An ultrastructural study
- PMID: 37095940
- PMCID: PMC10122028
- DOI: 10.1016/j.heliyon.2023.e15190
AL(light chain)-amyloidogenesis by mesangial cells involves active participation of lysosomes: An ultrastructural study
Abstract
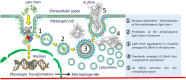
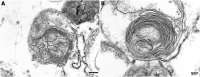
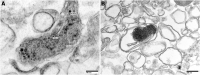
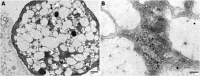
Amyloid formation by cells is a stepwise process that occurs in macrophages and cells capable of transforming into a macrophage phenotype. One such cell is the mesangial cell in the kidney. It has been shown that mesangial cells are engaged in AL (light chain associated)- amyloidogenesis after transforming phenotypically from a smooth muscle to a macrophage phenotype. The actual process of amyloid fibril formation has not been dissected. This ultrastructural study which includes the examination of lysosomal gradient specimens addresses this issue by analyzing the sequence of events that takes place as fibrils are formed in endosomes and lysosomes. The findings indicate that fibrillogenesis begins in endosomes but is completed and most pronounced in the lysosomal compartment. As early as 10 min after incubation of human mesangial cells with AL-LCs, amyloid fibrils are formed in endosomes but mostly occurs in the mature lysosomal compartment. This is the first time that fibril formation is demonstrated experimentally occurring inside human mesangial cells and the entire sequence of events taking place is elucidated.
Keywords: Amyloid; Amyloidogenesis; Endosomes; Gradient centrifugation; Immunoglobulin light chains; Kidney; Lysosomal gradient; Lysosomes; Mesangial cells.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The research was approved by the Institutional Board Review at the University of South Alabama (1,565,374–5).
Figures






Similar articles
-
Contribution of human smooth muscle cells to amyloid angiopathy in AL (light-chain) amyloidosis.Ultrastruct Pathol. 2017 Sep-Oct;41(5):358-368. doi: 10.1080/01913123.2017.1349852. Epub 2017 Aug 10. Ultrastruct Pathol. 2017. PMID: 28796568
-
In vitro AL-amyloid formation by rat and human mesangial cells.Lab Invest. 1996 Jan;74(1):290-302. Lab Invest. 1996. PMID: 8569193
-
Extrusion of amyloid fibrils to the extracellular space in experimental mesangial AL-amyloidosis: transmission and scanning electron microscopy studies and correlation with renal biopsy observations.Ultrastruct Pathol. 2014 Apr;38(2):104-15. doi: 10.3109/01913123.2013.861568. Epub 2014 Jan 24. Ultrastruct Pathol. 2014. PMID: 24460740
-
Understanding Mesangial Pathobiology in AL-Amyloidosis and Monoclonal Ig Light Chain Deposition Disease.Kidney Int Rep. 2020 Jul 21;5(11):1870-1893. doi: 10.1016/j.ekir.2020.07.013. eCollection 2020 Nov. Kidney Int Rep. 2020. PMID: 33163710 Free PMC article. Review.
-
[Molecular mechanism of amyloid formation by Ab peptide: review of own works].Biomed Khim. 2018 Jan;64(1):94-109. doi: 10.18097/PBMC20186401094. Biomed Khim. 2018. PMID: 29460839 Review. Russian.
Cited by
-
Role of the mechanisms for antibody repertoire diversification in monoclonal light chain deposition disorders: when a friend becomes foe.Front Immunol. 2023 Jul 13;14:1203425. doi: 10.3389/fimmu.2023.1203425. eCollection 2023. Front Immunol. 2023. PMID: 37520549 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

