Levels and durability of neutralizing antibodies against SARS-CoV-2 Omicron and other variants after ChAdOx-1 or BNT162b2 booster in CoronaVac-primed elderly individuals
- PMID: 37095993
- PMCID: PMC10116116
- DOI: 10.1016/j.heliyon.2023.e15653
Levels and durability of neutralizing antibodies against SARS-CoV-2 Omicron and other variants after ChAdOx-1 or BNT162b2 booster in CoronaVac-primed elderly individuals
Abstract
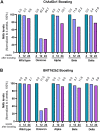
The outbreak of the SARS-CoV-2 Omicron variant raised the need for vaccine boosting. We evaluated the efficiency of the third booster vaccine, ChAdOx-1 or BNT162b2, in causing a neutralizing antibody (NAb) response and its durability against the Omicron and other variants in elderly individuals previously vaccinated with 2-dose CoronaVac inactivated vaccine. After receiving 2-dose CoronaVac, only 2.2% of subjects had NAbs against the Omicron variant above the cut-off value. Four weeks after boosting, the number of subjects who had NAb levels above the cut-off values in the ChAdOx-1 and BNT162b2 vaccine boosting groups increased to 41.7% and 54.5%, respectively. However, after 12 and 24 weeks of boosting with any vaccines, NAb levels against the Omicron variant dramatically waned. Twenty-four weeks after boosting, only 2% had high levels of NAbs against the Omicron variant. Compared to other variants, the Omicron variant was less responsive to boosting vaccines. The waning rate of NAb levels for the Omicron variant was much faster than that observed in the Alpha, Beta and Delta variants. To combat the Omicron variant, the fourth booster dose is, therefore, recommended for elderly individuals.
Keywords: Booster vaccine; COVID-19; Elderly; Neutralizing antibody; Omicron variant; SARS-CoV-2; Variants of concern.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Neutralizing antibody and T cell responses against SARS-CoV-2 variants of concern following ChAdOx-1 or BNT162b2 boosting in the elderly previously immunized with CoronaVac vaccine.Immun Ageing. 2022 May 24;19(1):24. doi: 10.1186/s12979-022-00279-8. Immun Ageing. 2022. PMID: 35610643 Free PMC article.
-
Immunogenicity, durability, and safety of an mRNA and three platform-based COVID-19 vaccines as a third dose following two doses of CoronaVac in China: A randomised, double-blinded, placebo-controlled, phase 2 trial.EClinicalMedicine. 2022 Sep 28;54:101680. doi: 10.1016/j.eclinm.2022.101680. eCollection 2022 Dec. EClinicalMedicine. 2022. PMID: 36188435 Free PMC article.
-
Boosting of serum neutralizing activity against the Omicron variant among recovered COVID-19 patients by BNT162b2 and CoronaVac vaccines.EBioMedicine. 2022 May;79:103986. doi: 10.1016/j.ebiom.2022.103986. Epub 2022 Apr 8. EBioMedicine. 2022. PMID: 35398786 Free PMC article.
-
Durability of immune response against omicron BA.2 and BA.4/5 and T cell responses after boosting with mRNA and adenoviral vector-based vaccines following heterologous CoronaVac/ChAdOx-1nCov-19 vaccination.Hum Vaccin Immunother. 2023 Dec 15;19(3):2283916. doi: 10.1080/21645515.2023.2283916. Epub 2023 Nov 28. Hum Vaccin Immunother. 2023. PMID: 38014687 Free PMC article.
-
The influence of Omicron on vaccine efficacy and durability: a neurology perspective.Clin Exp Vaccine Res. 2024 Jul;13(3):175-183. doi: 10.7774/cevr.2024.13.3.175. Epub 2024 Jul 31. Clin Exp Vaccine Res. 2024. PMID: 39144125 Free PMC article. Review.
Cited by
-
Neutralizing antibody response to different COVID-19 vaccines in Brazil: the impact of previous infection and booster doses.Front Immunol. 2025 Aug 4;16:1603612. doi: 10.3389/fimmu.2025.1603612. eCollection 2025. Front Immunol. 2025. PMID: 40831558 Free PMC article.
-
Immune Responses Against SARS-CoV-2 in Previously SARS-CoV-2-Infected Individuals after Receiving a Single Dose of CoronaVac or ChAdOx-1 Vaccine.Infect Chemother. 2025 Jun;57(2):274-287. doi: 10.3947/ic.2024.0145. Epub 2025 Apr 16. Infect Chemother. 2025. PMID: 40343423 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

