The safety of botulinum neurotoxin type A's intraarticular application in experimental animals
- PMID: 37096009
- PMCID: PMC10121478
- DOI: 10.1016/j.toxcx.2023.100155
The safety of botulinum neurotoxin type A's intraarticular application in experimental animals
Abstract
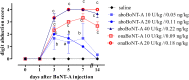
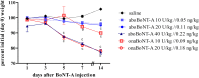
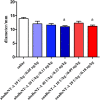
In vivo studies of botulinum neurotoxin type A (BoNT-A) enabled characterization of its activity in the nociceptive sensory system separate from its preferred action in motor and autonomic nerve terminals. However, in the recent rodent studies of arthritic pain which employed high intra-articular (i.a.) doses (expressed as a total number of units (U) per animal or U/kg), possible systemic effects have not been conclusively excluded. Herein we assessed the effect of two pharmaceutical preparations, abobotulinumtoxinA (aboBoNT-A, 10, 20, and 40 U/kg corresponding to 0.05, 0.11, and 0.22 ng/kg neurotoxin) and onabotulinumtoxinA (onaBoNT-A, 10 and 20 U/kg corresponding to 0.09 and 0.18 ng/kg, respectively) injected into the rat knee, on safety-relevant readouts: digit abduction, motor performance and weight gain during 14 days post-treatment. The i. a. toxin produced dose-dependent impairment of the toe spreading reflex and rotarod performance, which was moderate and transient after 10 U/kg onaBoNT-A and ≤20 U/kg aboBoNT-A doses, and severe and long-lasting (examined up to 14 days) after ≥20 U/kg of onaBoNT-A and 40 U/kg aboBoNT-A. In addition, lower toxin doses prevented the normal weight gain compared to controls, while higher doses induced marked weight loss (≥20 U/kg of onaBoNT-A and 40 U/kg aboBoNT-A). Commonly employed BoNT-A formulations, depending on the doses, cause local relaxation of the surrounding muscles and systemic adverse effects in rats. Thus, to evade possible toxin unwanted local or systemic spread, careful dosing and motor testing should be mandatory in preclinical behavioral studies, irrespective of the sites and doses of toxin application.
Keywords: Motor effects; Rats; Systemic spread; abobotulinumtoxinA; onabotulinumtoxinA.
©2023PublishedbyElsevierLtd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This study was supported by Ipsen, Croatian Science Foundation (Project ID: IP-2014-09-4503) and University of Zagreb Support project. Mikhail Kalinichev was an employee of Ipsen Innovation, France at the time the research was conducted. All other authors declare no conflicts of interest. The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Figures

Similar articles
-
Efficacy of abobotulinumtoxinA versus onabotulinumtoxinA for the treatment of refractory neurogenic detrusor overactivity: a systematic review and indirect treatment comparison.J Med Econ. 2023 Jan-Dec;26(1):200-207. doi: 10.1080/13696998.2023.2165366. J Med Econ. 2023. PMID: 36647624
-
The cost-effectiveness of abobotulinumtoxinA (Dysport) and onabotulinumtoxinA (Botox) for managing spasticity of the upper and lower limbs, and cervical dystonia.J Med Econ. 2022 Jan-Dec;25(1):919-929. doi: 10.1080/13696998.2022.2092354. J Med Econ. 2022. PMID: 35730362
-
Efficacy and Safety of AbobotulinumtoxinA for the Treatment of Glabellar Lines in Chinese Patients: A Pivotal, Phase 3, Randomized, Double-Blind and Open-Label Phase Study.Aesthetic Plast Surg. 2023 Feb;47(1):351-364. doi: 10.1007/s00266-022-03164-3. Epub 2022 Dec 19. Aesthetic Plast Surg. 2023. PMID: 36536093 Free PMC article. Clinical Trial.
-
Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats.Toxins (Basel). 2022 Feb 23;14(3):161. doi: 10.3390/toxins14030161. Toxins (Basel). 2022. PMID: 35324657 Free PMC article.
-
Evidence on botulinum toxin in selected disorders.Toxicon. 2018 Jun 1;147:134-140. doi: 10.1016/j.toxicon.2018.01.019. Epub 2018 Feb 3. Toxicon. 2018. PMID: 29408357 Review.
References
LinkOut - more resources
Full Text Sources

