Adeno-associated virus vector system controlling capsid expression improves viral quantity and quality
- PMID: 37096037
- PMCID: PMC10122016
- DOI: 10.1016/j.isci.2023.106487
Adeno-associated virus vector system controlling capsid expression improves viral quantity and quality
Abstract
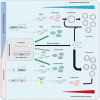
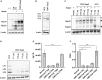
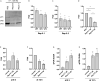
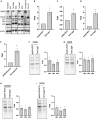
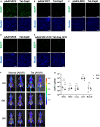
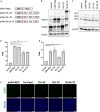
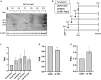
Adeno-associated virus (AAV) vectors are promising tools for gene therapy. The current AAV vector system produces an abundance of empty capsids that are eliminated before clinical use, leading to increased costs for gene therapy. In the present study, we established an AAV production system that regulates the timing of capsid expression using a tetracycline-dependent promoter. Tetracycline-regulating capsid expression increased viral yield and reduced empty capsids in various serotypes without altering AAV vector infectivity in vitro and in vivo. The replicase expression pattern change observed in the developed AAV vector system improved viral quantity and quality, whereas timing control of capsid expression reduced empty capsids. These findings provide a new perspective on the development of AAV vector production systems in gene therapy.
Keywords: Biotechnology; Virology.
© 2023 The Author(s).
Conflict of interest statement
T.E. and J.M. are the operating officer and director, respectively, and have treasury stocks in Takara Bio Inc. This work was partly funded by a Joint Research Fund sponsored by Takara Bio Inc. K.O. 1 and TAKARA bio Inc. have applied for the international patent based on present study. 1Kenji Ohba.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

