Adipocyte-derived chemerin rescues lipid overload-induced cardiac dysfunction
- PMID: 37096038
- PMCID: PMC10121453
- DOI: 10.1016/j.isci.2023.106495
Adipocyte-derived chemerin rescues lipid overload-induced cardiac dysfunction
Abstract
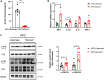
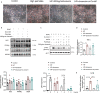
Chemerin, an adipocyte-secreted protein, has been recently suggested to be linked to metabolic syndrome and cardiac function in obese and diabetes mellitus. This study aimed to investigate the potential roles of adipokine chemerin on high fat-induced cardiac dysfunction. Chemerin (Rarres2) knockout mice, which were fed with either a normal diet or a high-fat diet for 20 weeks, were employed to observe whether adipokine chemerin affected lipid metabolism, inflammation, and cardiac function. Firstly, we found normal metabolic substrate inflexibility and cardiac function in Rarres2 -/- mice with a normal diet. Notably, in a high-fat diet, Rarres2 -/- mice showed lipotoxicity, insulin resistance, and inflammation, thus causing metabolic substrate inflexibility and cardiac dysfunction. Furthermore, by using in vitro model of lipid-overload cardiomyocytes, we found chemerin supplementation reversed the lipid-induced abnormalities above. Herein, in the presence of obesity, adipocyte-derived chemerin might function as an endogenous cardioprotective factor against obese-related cardiomyopathy.
Keywords: Cell biology; Cellular physiology; Physiology.
© 2023.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Chemerin deficiency regulates adipogenesis is depot different through TIMP1.Genes Dis. 2020 Apr 9;8(5):698-708. doi: 10.1016/j.gendis.2020.04.003. eCollection 2021 Sep. Genes Dis. 2020. PMID: 34291141 Free PMC article.
-
Atg7 Knockdown Reduces Chemerin Secretion in Murine Adipocytes.J Clin Endocrinol Metab. 2019 Nov 1;104(11):5715-5728. doi: 10.1210/jc.2018-01980. J Clin Endocrinol Metab. 2019. PMID: 31225870 Free PMC article.
-
Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice.J Endocrinol. 2014 Aug;222(2):201-15. doi: 10.1530/JOE-14-0069. Epub 2014 Jun 3. J Endocrinol. 2014. PMID: 24895415
-
The role of Chemerin in human diseases.Cytokine. 2023 Feb;162:156089. doi: 10.1016/j.cyto.2022.156089. Epub 2022 Dec 1. Cytokine. 2023. PMID: 36463659 Review.
-
Chemerin: a multifaceted adipokine involved in metabolic disorders.J Endocrinol. 2018 Aug;238(2):R79-R94. doi: 10.1530/JOE-18-0174. Epub 2018 May 30. J Endocrinol. 2018. PMID: 29848608 Free PMC article. Review.
Cited by
-
Adipokines and their potential impacts on susceptibility to myocardial ischemia/reperfusion injury in diabetes.Lipids Health Dis. 2024 Nov 13;23(1):372. doi: 10.1186/s12944-024-02357-w. Lipids Health Dis. 2024. PMID: 39538244 Free PMC article. Review.
-
Chemerin in the Spotlight: Revealing Its Multifaceted Role in Acute Myocardial Infarction.Biomedicines. 2024 Sep 20;12(9):2133. doi: 10.3390/biomedicines12092133. Biomedicines. 2024. PMID: 39335646 Free PMC article. Review.
-
Statins Prevent the Deleterious Consequences of Placental Chemerin Upregulation in Preeclampsia.Hypertension. 2024 Apr;81(4):861-875. doi: 10.1161/HYPERTENSIONAHA.123.22457. Epub 2024 Feb 15. Hypertension. 2024. PMID: 38361240 Free PMC article.
References
-
- Liu Y., Steinbusch L.K.M., Nabben M., Kapsokalyvas D., van Zandvoort M., Schönleitner P., Antoons G., Simons P.J., Coumans W.A., Geomini A., et al. Palmitate-induced vacuolar-type H(+)-ATPase inhibition feeds forward into insulin resistance and contractile dysfunction. Diabetes. 2017;66:1521–1534. doi: 10.2337/db16-0727. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

