Competitive survival of clonal serial Pseudomonas aeruginosa isolates from cystic fibrosis airways in human neutrophils
- PMID: 37096049
- PMCID: PMC10122015
- DOI: 10.1016/j.isci.2023.106475
Competitive survival of clonal serial Pseudomonas aeruginosa isolates from cystic fibrosis airways in human neutrophils
Abstract
Chronic airway infections with Pseudomonas aeruginosa are the major co-morbidity in most people with cystic fibrosis (CF) sustained by neutrophils as the major drivers of lung inflammation, damage, and remodeling. Phagocytosis assays were performed with clonal consortia of longitudinal P. aeruginosa airway isolates collected from people with CF since the onset of lung colonization until patient's death or replacement by another clone. The extra- and intracellular abundance of individual strains was assessed by deep amplicon sequencing of strain-specific single nucleotide variants in the bacterial genome. The varied microevolution of the accessory genome of the P. aeruginosa clones during mild and severe courses of infection corresponded with a differential persistence of clonal progeny in the neutrophil phagosome. By simultaneously exposing the ancestor and its progeny to the same habitat, the study recapitulated the time lapse of the temporal change of the fitness of the clone to survive in neutrophils.
Keywords: Immunology; Microbiology; Respiratory medicine.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



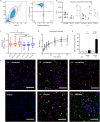


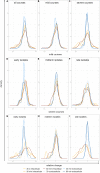
Similar articles
-
Intraclonal competitive fitness of longitudinal cystic fibrosis Pseudomonas aeruginosa airway isolates in liquid cultures.Environ Microbiol. 2020 Jul;22(7):2536-2549. doi: 10.1111/1462-2920.14924. Epub 2020 Feb 7. Environ Microbiol. 2020. PMID: 31985137
-
Competitive fitness of Pseudomonas aeruginosa isolates in human and murine precision-cut lung slices.Front Cell Infect Microbiol. 2022 Aug 23;12:992214. doi: 10.3389/fcimb.2022.992214. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36081773 Free PMC article.
-
Microevolution of Pseudomonas aeruginosa in the airways of people with cystic fibrosis.Curr Opin Immunol. 2023 Aug;83:102328. doi: 10.1016/j.coi.2023.102328. Epub 2023 Apr 26. Curr Opin Immunol. 2023. PMID: 37116385 Review.
-
Long-Term Microevolution of Pseudomonas aeruginosa Differs between Mildly and Severely Affected Cystic Fibrosis Lungs.Am J Respir Cell Mol Biol. 2018 Aug;59(2):246-256. doi: 10.1165/rcmb.2017-0356OC. Am J Respir Cell Mol Biol. 2018. PMID: 29470920
-
Infections with Pseudomonas aeruginosa in patients with cystic fibrosis.Behring Inst Mitt. 1997 Feb;(98):249-55. Behring Inst Mitt. 1997. PMID: 9382747 Review.
Cited by
-
Mucin adhesion of serial cystic fibrosis airways Pseudomonas aeruginosa isolates.Front Cell Infect Microbiol. 2024 Aug 22;14:1448104. doi: 10.3389/fcimb.2024.1448104. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39239637 Free PMC article.
References
LinkOut - more resources
Full Text Sources

