A Randomized Clinical Trial of Transgender Women Switching to B/F/TAF: The (mo)BETTA Trial
- PMID: 37096146
- PMCID: PMC10122488
- DOI: 10.1093/ofid/ofad178
A Randomized Clinical Trial of Transgender Women Switching to B/F/TAF: The (mo)BETTA Trial
Abstract
Background: Cardiometabolic disease in transgender women (TW) is affected by gender-affirming hormonal therapies (GAHTs), HIV, and antiretroviral therapy (ART). We evaluated the 48-week safety/tolerability of switching to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) vs continued ART in TW on GAHT.
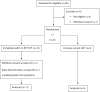
Methods: TW on GAHT and suppressive ART were randomized 1:1 to switch to B/F/TAF (Arm A) or continue current ART (Arm B). Cardiometabolic biomarkers, sex hormones, bone mineral density (BMD) and lean/fat mass by DXA scan, and hepatic fat (controlled continuation parameter [CAP]) were measured. Wilcoxon rank-sum/signed-rank and χ2 tests compared continuous and categorical variables.
Results: TW (Arm A n = 12, Arm B n = 9) had a median age of 45 years. Ninety-five percent were non-White; 70% were on elvitegravir or dolutegravir, 57% TAF, 24% abacavir, and 19% TDF; 29% had hypertension, 5% diabetes, and 62% dyslipidemia. There were no adverse events. Arm A/B had 91%/89% undetectable HIV-1 RNA at week 48 (w48). Baseline (BL) osteopenia (Arm A/B 42%/25%) and osteoporosis (17%/13%) were common, without significant changes. BL lean/fat mass were similar. At w48, Arm A had stable lean mass but increased limb (3 lbs) and trunk (3 lbs) fat (within-arm P < .05); fat in Arm B remained stable. No changes occurred in lipid or glucose profiles. Arm B had a greater w48 decrease (-25 vs -3 dB/m; P = .03) in CAP. BL and w48 concentrations of all biomarkers were similar.
Conclusions: In this cohort of TW, switch to B/F/TAF was safe and metabolically neutral, though greater fat gain occurred on B/F/TAF. Further study is needed to better understand cardiometabolic disease burden in TW with HIV.
Keywords: HIV; bictegravir; fat; randomized clinical trial; transgender women.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J.E.L. has received research support from Gilead Sciences related and unrelated to the work, research support from CytoDyn, Pfizer, and OncoImmune unrelated to the work, and consultancy support from Gilead Sciences, Merck and Co., and Theratechnologies (consultancy unrelated to the work). A.N.H.: No conflict. H.F.: No conflict. P.D.: No conflict. A.K.: No conflict. H.M.: No conflict. L.P.: No conflict. S.B.: No conflict. S.B.: No conflict. N.R.: No conflict. T.T.B. has served as a consultant to Merck, Gilead Sciences, ViiV Healthcare, Janssen, and Theratechnologies unrelated to the work. N.F. has served as a consultant for Gilead and has received research support from Gilead for unrelated work. All other authors report no potential conflicts.
Figures
References
-
- Centers for Disease Control and Prevention . HIV and transgender people: HIV prevalence 2019–2020. Available at:https://www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html. Accessed Feb 28, 2023.
-
- Lake JE, Currier JS. Metabolic disease in HIV infection. Lancet Infect Dis 2013; 13:964–75. - PubMed
-
- Klaver M, de Blok CJM, Wiepjes CM, et al. . Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol 2018; 178:163–71. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous