Effects of hydrogen gas inhalation on L-DOPA-induced dyskinesia
- PMID: 37096172
- PMCID: PMC10121822
- DOI: 10.1016/j.bbih.2023.100623
Effects of hydrogen gas inhalation on L-DOPA-induced dyskinesia
Abstract
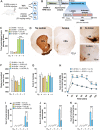
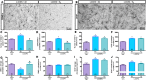
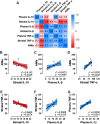
L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia is a side effect of Parkinson's disease treatment and it is characterized by atypical involuntary movements. A link between neuroinflammation and L-DOPA-induced dyskinesia has been documented. Hydrogen gas (H2) has neuroprotective effects in Parkinson's disease models and has a major anti-inflammatory effect. Our objective is to test the hypothesis that H2 inhalation reduces L-DOPA-induced dyskinesia. 15 days after 6-hydroxydopamine lesions of dopaminergic neurons were made (microinjection into the medial forebrain bundle), chronic L-DOPA treatment (15 days) was performed. Rats were exposed to H2 (2% gas mixture, 1 h) or air (controls) before L-DOPA injection. Abnormal involuntary movements and locomotor activity were conducted. Striatal microglia and astrocyte was analyzed and striatal and plasma samples for cytokines evaluation were collected after the abnormal involuntary movements analysis. H2 inhalation attenuated L-DOPA-induced dyskinesia. The gas therapy did not impair the improvement of locomotor activity achieved by L-DOPA treatment. H2 inhalation reduced activated microglia in the lesioned striatum, which is consistent with the observed reduced pro-inflammatory cytokines levels. Display of abnormal involuntary movements was positively correlated with plasma IL-1β and striatal TNF-α levels and negatively correlated with striatal IL-10 levels. Prophylactic H2 inhalation decreases abnormal involuntary movements in a preclinical L-DOPA-induced dyskinesia model. The H2 antidyskinetic effect was associated with decreased striatal and peripheral inflammation. This finding has a translational importance to L-DOPA-treated parkinsonian patients' well-being.
Keywords: 6-Hydroxydopamine; Neuroinflammation; Parkinson's disease; Striatum; Systemic inflammation.
©2023PublishedbyElsevierInc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
GABA storage and release in the medial globus pallidus in L-DOPA-induced dyskinesia priming.Neurobiol Dis. 2020 Sep;143:104979. doi: 10.1016/j.nbd.2020.104979. Epub 2020 Jun 24. Neurobiol Dis. 2020. PMID: 32590036
-
Differential induction of dyskinesia and neuroinflammation by pulsatile versus continuous l-DOPA delivery in the 6-OHDA model of Parkinson's disease.Exp Neurol. 2016 Dec;286:83-92. doi: 10.1016/j.expneurol.2016.09.013. Epub 2016 Sep 30. Exp Neurol. 2016. PMID: 27697481
-
Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat.Exp Neurol. 1999 Feb;155(2):204-20. doi: 10.1006/exnr.1998.6996. Exp Neurol. 1999. PMID: 10072296
-
L-DOPA-induced dyskinesia, is striatal dopamine depletion a requisite?J Neurol Sci. 2015 Apr 15;351(1-2):9-12. doi: 10.1016/j.jns.2015.02.041. Epub 2015 Mar 1. J Neurol Sci. 2015. PMID: 25758471 Review.
-
Animal models of l-dopa-induced dyskinesia in Parkinson's disease.Mov Disord. 2018 Jul;33(6):889-899. doi: 10.1002/mds.27337. Epub 2018 Feb 28. Mov Disord. 2018. PMID: 29488257 Review.
Cited by
-
Levodopa-induced dyskinesia: interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation.Front Immunol. 2023 Oct 4;14:1253273. doi: 10.3389/fimmu.2023.1253273. eCollection 2023. Front Immunol. 2023. PMID: 37860013 Free PMC article. Review.
-
Inhaled molecular hydrogen reduces hippocampal neuroinflammation, glial reactivity and ameliorates memory impairment during systemic inflammation.Brain Behav Immun Health. 2023 Jun 17;31:100654. doi: 10.1016/j.bbih.2023.100654. eCollection 2023 Aug. Brain Behav Immun Health. 2023. PMID: 37449286 Free PMC article.
References
-
- Amorim M.R., et al. Increased lipopolysaccharide-induced hypothermia in neurogenic hypertension is caused by reduced hypothalamic PGE 2 production and increased heat loss. J. Physiol. 2020;598:4663–4680. - PubMed
-
- Amri F., Ghouili I., Amri M., Carrier A., Masmoudi-Kouki O. Neuroglobin protects astroglial cells from hydrogen peroxide-induced oxidative stress and apoptotic cell death. J. Neurochem. 2017;140:151–169. - PubMed
LinkOut - more resources
Full Text Sources

