Gumarontinib in patients with non-small-cell lung cancer harbouring MET exon 14 skipping mutations: a multicentre, single-arm, open-label, phase 1b/2 trial
- PMID: 37096188
- PMCID: PMC10121392
- DOI: 10.1016/j.eclinm.2023.101952
Gumarontinib in patients with non-small-cell lung cancer harbouring MET exon 14 skipping mutations: a multicentre, single-arm, open-label, phase 1b/2 trial
Abstract
Background: Approximately 3-4% of patients with non-small-cell lung cancer (NSCLC) have MET exon 14 (METex14) skipping mutations. We report primary results from the phase 2 stage of a phase 1b/2 study of gumarontinib, a selective, potent, oral MET inhibitor, in patients with METex14 skipping mutation-positive (METex14-positive) NSCLC.
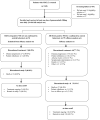
Methods: The single-arm, multicentre, open-label, phase 2 stage of the GLORY study was conducted at 42 centres across China and Japan. Adults with locally advanced or metastatic METex14-positive NSCLC received oral gumarontinib 300 mg once daily in continuous 21-day cycles until disease progression, intolerable toxicity, or withdrawal of consent. Eligible patients had failed one or two prior lines of therapy (not including a MET inhibitor), were ineligible for/refused chemotherapy, and had no genetic alterations targetable with standard therapies. The primary endpoint was objective response rate in patients with a valid baseline tumour assessment, by blinded independent review. The study was registered at ClinicalTrials.gov (NCT04270591).
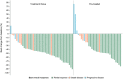
Findings: Between Aug 2, 2019 and Apr 28, 2021, 84 patients were enrolled and received gumarontinib (median follow-up 13.5 months [IQR 8.7-17.1]), at data cut-off (Apr 28, 2022) five patients whose METex14 status could not be confirmed by a central laboratory were excluded from the efficacy analysis. The objective response rate was 66% (95% CI 54-76) overall (n = 79), 71% (95% CI 55-83) in treatment-naïve patients (n = 44), and 60% (95% CI 42-76) in previously-treated patients (n = 35). The most common treatment-related adverse events (any grade) were oedema (67/84 patients, 80%) and hypoalbuminuria (32/84, 38%). Grade ≥3 treatment-emergent adverse events occurred in 45 (54%) patients. Treatment-related adverse events leading to permanent discontinuation occurred in 8% (7/84) of patients.
Interpretation: Gumarontinib monotherapy had durable antitumour activity with manageable toxicity in patients with locally advanced or metastatic METex14-positive NSCLC when used in first line or later.
Funding: Haihe Biopharma Co., Ltd. Supported in part by grants from the National Science and Technology Major Project of China for "Clinical Research of Gumarontinib, a highly selective MET inhibitor" (2018ZX09711002-011-003); the National Natural Science Foundation of China (82030045 to S.L. and 82172633 to YF.Y); Shanghai Municipal Science & Technology Commission Research Project (19411950500 to S.L.); Shanghai Shenkang Action Plan (16CR3005A to S.L.) and Shanghai Chest Hospital Project of Collaborative Innovation (YJXT20190105 to S.L.).
Keywords: Lung cancer; MET inhibitor; MET mutation.
© 2023 The Author(s).
Conflict of interest statement
G-K reports grants from Haihe Biopharma Co., Ltd., during the conduct of the study; grants and personal fees from Amgen Inc., grants and personal fees from Amgen K.K., personal fees from Amoy Diagnosties Co., Ltd., grants from Amgen Astellas BioPharma K.K., grants and personal fees from AstraZeneca K.K., personal fees from Bayer U.S., grants from Bayer Yakuhin, Ltd., grants from Boehringer Ingelheim Japan, Inc., grants and personal fees from Bristol-Myers Squibb K.K., grants from Blueprint Medicines Corporation, grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Daiichi Sankyo Co., Ltd., grants and personal fees from Eisai Co., Ltd., grants and personal fees from Eli Lilly Japan K.K., personal fees from Guardant Health Inc., grants from Ignyta, Inc., grants and personal fees from Janssen Pharmaceutical K.K., grants from Kissei Pharmaceutical Co., Ltd., grants from Kyowa Kirin Co., Ltd., personal fees from Life Technologies Japan Ltd., grants from Loxo Oncology, Inc., grants from Medical & Biological Laboratories Co., Ltd., personal fees from Medpace Japan K.K., grants and personal fees from Merck Biopharma Co., Ltd., grants from Merus N.V., grants and personal fees from MSD K.K., grants from NEC Corporation, grants and personal fees from Novartis Pharma K.K., grants and personal fees from Ono Pharmaceutical Co., Ltd., grants from Pfizer Japan Inc., grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Spectrum Pharmaceuticals, Inc., grants from Sysmex Corporation, grants and personal fees from Taiho Pharmaceutical Co., Ltd., grants and personal fees from Takeda Pharmaceutical Co., Ltd., grants from Turning Point Therapeutics, Inc., outside the submitted work. N-K reports grants from Haihe Biopharma Co., Ltd., during the conduct of the study; grants from ONO Pharmaceutical Co., Ltd., grants and personal fees from MSD, grants from Taiho Pharmaceutical Co., Ltd., grants from AbbVie, grants from Daiichi Sankyo Company, Limited, grants from Amgen, grants from Eisai Co., Ltd., grants from Sanofi K.K., grants from Janssen Pharmaceutical K.K., grants from Novartis Pharmaceuticals, grants from Pfizer, grants from Eli Lilly Japan, grants and personal fees from Merck Biopharma Co., Ltd., grants and personal fees from Takeda Pharmaceutical Co., Ltd., grants and personal fees from Chugai pharmaceutical, grants from Merus, grants and personal fees from AstraZeneca, personal fees from Nippon Boehringer Ingelheim, personal fees from Roche Diagnostics, personal fees from Bristol Myers Squibb, personal fees from Nippon Kayaku, outside the submitted work. S-J reports grants from Eli Lilly Japan K.K., grants from Nippon Boehringer Ingelheim Co., Ltd., outside the submitted work. Y-Y reports ‘other’ interests in Taiho Pharmaceutical Co., Ltd., ‘other’ from Takeda Pharmaceutical Company Limited., ‘other’ from Eisai Co., Ltd., outside the submitted work. L-FG, Z-J, L-M, W-QX, and S-MH report personal fees from Haihe Biopharma Co., Ltd, during the conduct of the study; personal fees from Haihe Biopharma Co., Ltd, outside the submitted work. L-S reports grants from Haihe Biopharma Co., Ltd., outside the submitted work. All other authors declare no competing interests.
Figures

Similar articles
-
Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study.Lancet Respir Med. 2021 Oct;9(10):1154-1164. doi: 10.1016/S2213-2600(21)00084-9. Epub 2021 Jun 21. Lancet Respir Med. 2021. PMID: 34166627 Clinical Trial.
-
Savolitinib in patients in China with locally advanced or metastatic treatment-naive non-small-cell lung cancer harbouring MET exon 14 skipping mutations: results from a single-arm, multicohort, multicentre, open-label, phase 3b confirmatory study.Lancet Respir Med. 2024 Dec;12(12):958-966. doi: 10.1016/S2213-2600(24)00211-X. Epub 2024 Sep 10. Lancet Respir Med. 2024. PMID: 39270695 Clinical Trial.
-
A pooled analysis of clinical outcome in driver-gene negative non-small cell lung cancer patients with MET overexpression treated with gumarontinib.Ther Adv Med Oncol. 2024 Jul 31;16:17588359241264730. doi: 10.1177/17588359241264730. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39091606 Free PMC article.
-
Capmatinib for patients with non-small cell lung cancer with MET exon 14 skipping mutations: A review of preclinical and clinical studies.Cancer Treat Rev. 2021 Apr;95:102173. doi: 10.1016/j.ctrv.2021.102173. Epub 2021 Mar 1. Cancer Treat Rev. 2021. PMID: 33740553 Review.
-
Safety of MET Tyrosine Kinase Inhibitors in Patients With MET Exon 14 Skipping Non-small Cell Lung Cancer: A Clinical Review.Clin Lung Cancer. 2022 May;23(3):195-207. doi: 10.1016/j.cllc.2022.01.003. Epub 2022 Feb 4. Clin Lung Cancer. 2022. PMID: 35272955 Review.
Cited by
-
Targeting the MET gene: unveiling therapeutic opportunities in immunotherapy within the tumor immune microenvironment of non-small cell lung cancer.Ther Adv Med Oncol. 2024 Oct 17;16:17588359241290733. doi: 10.1177/17588359241290733. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39483139 Free PMC article. Review.
-
Targeting MET in NSCLC: An Ever-Expanding Territory.JTO Clin Res Rep. 2024 Jan 3;5(2):100630. doi: 10.1016/j.jtocrr.2023.100630. eCollection 2024 Feb. JTO Clin Res Rep. 2024. PMID: 38361739 Free PMC article. Review.
-
The continually evolving landscape of novel therapies in oncogene-driven advanced non-small-cell lung cancer.Ther Adv Med Oncol. 2025 Jan 7;17:17588359241308784. doi: 10.1177/17588359241308784. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 39776537 Free PMC article. Review.
-
Molecular mechanism of type ib MET inhibitors and their potential for CNS tumors.Sci Rep. 2025 Feb 26;15(1):6926. doi: 10.1038/s41598-025-85631-w. Sci Rep. 2025. PMID: 40011494 Free PMC article.
-
[Drug Resistance Mechanism and Therapeutic Strategy of Targeted Therapy of Non-small Cell Lung Cancer with MET Alterations].Zhongguo Fei Ai Za Zhi. 2023 Sep 20;26(9):684-691. doi: 10.3779/j.issn.1009-3419.2023.102.33. Zhongguo Fei Ai Za Zhi. 2023. PMID: 37985154 Free PMC article. Chinese.
References
-
- Gentile A., Trusolino L., Comoglio P.M. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27(1):85–94. - PubMed
-
- Reungwetwattana T., Liang Y., Zhu V., Ou S.I. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: the Why, the How, the Who, the Unknown, and the Inevitable. Lung Cancer. 2017;103:27–37. - PubMed
-
- Frampton G.M., Ali S.M., Rosenzweig M., et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–859. - PubMed
-
- Vuong H.G., Ho A.T.N., Altibi A.M.A., Nakazawa T., Katoh R., Kondo T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer - a systematic review and meta-analysis. Lung Cancer. 2018;123:76–82. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

