In situ self-assembled organoid for osteochondral tissue regeneration with dual functional units
- PMID: 37096194
- PMCID: PMC10121637
- DOI: 10.1016/j.bioactmat.2023.04.002
In situ self-assembled organoid for osteochondral tissue regeneration with dual functional units
Abstract
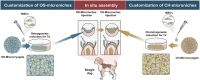
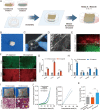
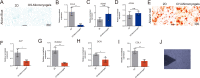
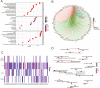
The regeneration of hierarchical osteochondral units is challenging due to difficulties in inducing spatial, directional and controllable differentiation of mesenchymal stem cells (MSCs) into cartilage and bone compartments. Emerging organoid technology offers new opportunities for osteochondral regeneration. In this study, we developed gelatin-based microcryogels customized using hyaluronic acid (HA) and hydroxyapatite (HYP), respectively for inducing cartilage and bone regeneration (denoted as CH-Microcryogels and OS-Microcryogels) through in vivo self-assembly into osteochondral organoids. The customized microcryogels showed good cytocompatibility and induced chondrogenic and osteogenic differentiation of MSCs, while also demonstrating the ability to self-assemble into osteochondral organoids with no delamination in the biphasic cartilage-bone structure. Analysis by mRNA-seq showed that CH-Microcryogels promoted chondrogenic differentiation and inhibited inflammation, while OS-Microcryogels facilitated osteogenic differentiation and suppressed the immune response, by regulating specific signaling pathways. Finally, the in vivo engraftment of pre-differentiated customized microcryogels into canine osteochondral defects resulted in the spontaneous assembly of an osteochondral unit, inducing simultaneous regeneration of both articular cartilage and subchondral bone. In conclusion, this novel approach for generating self-assembling osteochondral organoids utilizing tailor-made microcryogels presents a highly promising avenue for advancing the field of tissue engineering.
Keywords: Mesenchymal stem cells; Microcryogels; Organoid; Osteochondral regeneration; Self-assembly.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures










References
LinkOut - more resources
Full Text Sources

