Standing-up COVID-19 monoclonal antibody infusion centers: Infection prevention and control
- PMID: 37096642
- PMCID: PMC9485416
- DOI: 10.1016/j.ajic.2022.09.021
Standing-up COVID-19 monoclonal antibody infusion centers: Infection prevention and control
Abstract
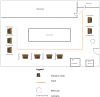
This paper describes the creation of outpatient monoclonal antibody (mAb) infusion centers for COVID-19 patients in a large academic medical center. It shows how the early and consistent partnership between infection prevention and the clinical and operational teams to establish and implement policies and procedures led to efficient and safe workflows.
Keywords: Emergency use authorization; Prophylaxis.
Copyright © 2022 Association for Professionals in Infection Control and Epidemiology, Inc. Published by Elsevier Inc. All rights reserved.
References
-
- FDA Authorizes REGEN-COV Monoclonal Antibody Therapy for Post-exposure Prophylaxis (prevention) for COVID-19. FDA. [online] (2021). Accessed July 13, 2021.https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-re...
-
- FDA Authorizes Bamlanivimab and Etesevimab Monoclonal Antibody Therapy for Post-exposure Prophylaxis (prevention) for COVID-19. FDA. [online] (2021). Accessed July 13, 2021.https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-ba...